In Review
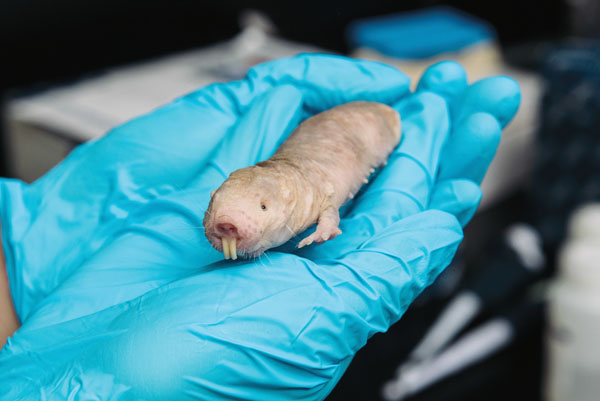 CANCER-PROOF: Naked mole rats have never been known to get cancer, despite a 30-year lifespan. (Photo: Brandon Vick)
CANCER-PROOF: Naked mole rats have never been known to get cancer, despite a 30-year lifespan. (Photo: Brandon Vick)What makes naked mole rats cancer-proof? Two Rochester biologists may have the answer, and their discovery could lead to new ways of treating cancer.
The exotic subterranean rodents have never been known to get cancer, despite a 30-year lifespan. A research group—led by Vera Gorbunova, professor of biology, and Andrei Seluanov, assistant professor of biology—has discovered that the tissues of the animals are rich with a chemical called high molecular weight hyaluronan, or HMW-HA, a form of a chemical that’s known to make tissues supple and aid in the healing process. The study was published in the journal Nature.
In 2009, the team discovered that mole rats’ cells stop proliferating when too many of them crowd together, cutting off runaway growth before it can start. That’s due to the cells’ expression of a gene called P16. Now the scientists have identified HMW-HA as the chemical that activates the anticancer response of the P16 gene. The biologists speculate that the rodents developed higher levels of the chemical in their skin to adapt to life in underground tunnels.
Gorbunova and Seluanov also identified the gene—called Has2—responsible for making HMW-HA in the naked mole rat. Surprisingly, the gene was different from the same gene in all other animals. Naked mole rats are also very slow at recycling HMW-HA, which contributes to the accumulation of the chemical in the mole rats’ tissues.
If further tests go well, the team hopes to try the chemical on human cells. HMW-HA is already used in antiwrinkle treatments and injections to ease arthritis pain in knee joints, without adverse effects. “Our hope is that it can also induce an anticancer response,” says Seluanov.
—Peter Iglinski
Scientists Coax Brain to Regenerate Cells Lost in Huntington’s Disease
Replenishing a type of neuron lost in Huntington’s disease may be possible using the brain’s native stem cells.
That’s according to a study published in the journal Cell Stem Cell, in which Medical Center scientists both triggered the production of new neurons in mice with the disease and showed that the new cells successfully integrated into the brain’s existing neural networks, dramatically extending the survival of the treated mice.
The study points to the feasibility of a new approach to treating Huntington’s disease, says Steven Goldman, professor of neurology and codirector of the University’s Center for Translational Neuromedicine.
Huntington’s is an inherited neurodegenerative disease characterized by the loss of a kind of cell called a medium spiny neuron, which is critical to motor control. The disease affects some 30,000 people in the United States and results in involuntary movements, coordination problems, and ultimately, cognitive decline and depression. Currently no treatment exists to slow or modify the fatal disease.
Shortly after birth, humans’ neural stem cells stop generating neurons and instead produce glia, a family of support cells that pervade the central nervous system. Some parts of the human brain—such as the hippocampus, where memories are formed and stored—produce neurons into adulthood. But in the striatum, the brain region devastated by Huntington’s disease, this capability is “switched off” in adulthood.
For the past decade, Goldman and his team have attempted to determine the precise chemical responsible for telling neural stem cells when to create neurons or glia cells. Through their research on brain plasticity in canaries, the team realized that by combining a protein with another molecule, they could switch the molecular machinery of stem cells from glial production and over to the production of neurons.
Researchers were able to extend significantly the survival of the treated mice, in some cases doubling their life expectancy.
—Mark Michaud
Socioeconomic Status Plays Major Role in Opioid Pain Control
Patients in moderate to severe pain in emergency rooms across the United States are less likely to receive opioid pain medications if they’re black, Hispanic, poor, or have less education, according to a new study co-authored by Michael Joynt ’12M (MD), now a pediatric cardiology fellow at the University of Michigan, and Meghan Train, a resident in the Department of Medicine.
Racial and ethnic disparities are well documented in the scientific literature, but the team believes the work is the first to investigate whether aspects of socioeconomic status—poverty, income, and education levels—also influence the prescription of opioid pain medications.
The results point to a need for a national discussion to increase awareness and to provide consistent, unbiased treatments, says corresponding author Robert Fortuna, assistant professor of medicine and pediatrics.
Investigators analyzed a cross-section of data from the National Hospital Ambulatory Care Survey of people 18 and older from 2006 to 2009. In more than 50,000 emergency room visits,
atients in the highest-income neighborhoods received prescriptions 49 percent of the time for moderate to severe pain, versus 39 percent of the time for patients from lower-income areas. Discrepancies also existed among various levels of poverty, with the poorest least likely to get opioids for pain. The study was published in the Journal of General Internal Medicine. —
Leslie Orr
Brain’s ‘Garbage Truck’ May Hold Key to Treating Alzheimer’s and Other Disorders
The brain’s system of waste removal, newly discovered by researchers, is a potentially powerful tool to treat neurological disorders like Alzheimer’s disease, says Maiken Nedergaard, the Frank P. Smith Professor of Neurosurgery. In fact, scientists believe that some disorders may arise when the system isn’t doing its job properly.
“Essentially all neurodegenerative diseases are associated with the accumulation of cellular waste products,” Nedergaard, codirector of the Center for Translational Medicine, writes in the journal Science about how modulating the brain’s system for removing toxic waste could lead to new treatments.
The body defends the brain like a fortress and rings it with a system of gateways that control which molecules can enter and exit. Though this blood-brain barrier was first described in the late 19th century, scientists are only beginning to understand the dynamics of how it functions. The complex network of waste removal—which researchers have dubbed the glymphatic system—was first disclosed by Nedergaard and colleagues last August in the journal Science Translational Medicine.
The key to discovering and understanding the system was the advent of a new imaging technology called two-photon microscopy, which allows scientists to peer deep within the living brain.
Understanding how the brain removes waste—both effectively and when the system breaks down—could have significant implications for treating neurological disorders. A hallmark of Alzheimer’s disease is the accumulation in the brain of the protein beta amyloid. Over time, the proteins amass with such density that they can be observed as plaques on scans of the brain.
“The idea that ‘dirty brain’ diseases like Alzheimer’s may result from a slowing down of the glymphatic system as we age is a completely new way to think about neurological disorders,” Nedergaard says. “It also presents us with a new set of targets to potentially increase the efficiency of glymphatic clearance and, ultimately, change the course of these conditions.”
—Mark Michaud

