Features
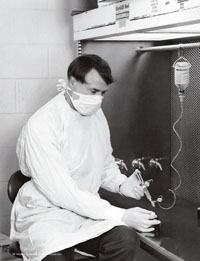
 AIMING HIGH: “We were really aiming at something that American pediatricians were looking for,” says Porter Anderson, a Rochester biochemist who helped develop the first vaccine for toddlers and infants that was effective against a form of bacterial meningitis. (Photo: Medical Center Communications)
AIMING HIGH: “We were really aiming at something that American pediatricians were looking for,” says Porter Anderson, a Rochester biochemist who helped develop the first vaccine for toddlers and infants that was effective against a form of bacterial meningitis. (Photo: Medical Center Communications)Nina Schor remembers the sharp demarcation between the prevaccine and postvaccine eras in the pediatric history of Haemophilus influenzae type b, an infectious bacterium better known by its initials, Hib.
There were the little details, like the smell. As a resident at Boston Children’s Hospital in the 1980s, Schor remembers the infant and toddler ward filled with children being treated with the antibiotic ampicillin to fight off the infections caused by Hib. At the worst, those include not only meningitis, but also pneumonia, arthritis, and epiglottitis—a life-threatening infection of the throat.
The diapers on the ward would take on a readily recognizable odor as the kids excreted ampicillin in their urine.
“We used to joke that as you got off the elevator on Division 26 of Boston Children’s, you could smell the ampicillin in the air,” says Schor, now the pediatrician-in-chief at Golisano Children’s Hospital and the William H. Eilinger Chair of Pediatrics. “I mean, the whole ward smelled from the diapers of these babies.”
By 1994, when she was an associate professor of child neurology at the University of Pittsburgh, the infections were so rare that a single case of a six-month-old admitted with meningitis caused by Hib became an important teaching moment for medical students and residents at the pediatric intensive care unit at Children’s Hospital of Pittsburgh.
“Before the cultures came back and the gram stain was done, we—the intensivist, the general pediatrician, and I—had made the diagnosis because the kid clearly had meningitis and was anemic,” Schor says. “And so we said, ‘Gee, we haven’t seen this in half a dozen years, but this looks like Haemophilus influenzae.’ And sure enough, that’s what it was.
“And the next thing we knew, it seemed like a hundred students and residents who had never in their lives seen a case of Haemophilus meningitis came down to the PICU to see this very unusual baby.”
“It seemed like overnight that it went from seeing this as the predominant disease on the infant and toddler ward to not seeing it at all,” Schor says. “I’m sure there was a middle zone in which it transitioned, but it just seemed to me to happen very rapidly.”
A key point in that history can be traced to a makeshift lab in the School of Medicine and Dentistry. There, at the time when Schor was a resident on the wards of Boston Children’s Hospital, a Medical Center team was refining an approach to vaccine technology that helped launch a new era in pediatric medicine.
Led by the pediatrician David Smith, a team including chemist Porter Anderson, now professor emeritus of pediatrics, and former Medical Center pediatric immunologist Richard Insel developed their version of “conjugate vaccine technology,” an approach to boosting the immunity-inducing power of vaccines that’s credited with nearly eradicating a once widely feared childhood infection.
The U.S. Food and Drug Administration approved the Rochester vaccine for use in infants in the fall of 1990. (A different version, based on the work of NIH scientists Rachel Schneerson and John Robbins, had already been approved for toddlers.)
Steven Cochi, a senior advisor to the director of the Global Immunization Division at the Centers for Disease Control and Prevention, says the Rochester work was a “big game changer.”
“The disease virtually disappeared because the vaccine worked,” say Cochi, himself a pediatrician who has tracked the incidence of Hib infections for more than 30 years. “And that’s the message here: vaccines work, and we need to use them.”
According to CDC figures, before the advent of an effective vaccine, there were roughly 20,000 Hib infections annually in the United States, primarily among children younger than five. By 2011, only 14 Hib infections in children younger than five were reported in the country. “It has become a rare disease in the United States,” says Cochi.
While the original Rochester vaccine was eventually superseded, the technology behind it was licensed for what became a highly effective vaccine against pneumococcal meningitis, an equally dangerous childhood infection that has also become rare in the United States. Far and away the most lucrative in Rochester’s history, the agreement has accounted for more than $150 million in licensing revenue.
Anderson, who received an honorary doctor of science degree at the School of Medicine and Dentistry’s commencement ceremony last spring, says he was drawn to solving a complicated biochemical question when Smith approached him about working on a vaccine. The two originally had met at Boston Children’s Hospital, where Smith’s training as a physician had prompted the idea of developing a Hib vaccine.
Lacking a background in chemistry, Smith turned to Anderson. When Smith joined the Rochester faculty, he recruited Anderson to join his small team.
“We were really aiming at something that American pediatricians were looking for, or hoping for, and that they needed,” Anderson says. “Such was state-of-the-art pediatric care in the U.S. that Haemophilus meningitis was something that scared the heck out of pediatricians. I learned about this through David Smith.”
Throughout his career, Anderson has described himself as a “Yankee engineer”—“I like solving problems with the materials at hand more than framing hypotheses,” he has written. He credits the entrepreneurial spirit of the Medical Center with fostering an atmosphere in which a small group of physicians and research scientists could work collaboratively on a problem. That wasn’t always the case in medical research at the time, he says.
Setting up a lab in the Medical Center, the team worked through iterations of the vaccine, developing an initial version that used a surface carbohydrate molecule from Hib bacteria to effectively induce immunity in toddlers.
But that early version was not as effective in infants, the most at-risk population. When Smith found that he couldn’t interest a pharmaceutical company in commercializing their research, the three established their own start-up, Praxis Biologics.
Working from a small “clean room” in what is now the Larry and Cindy Bloch Alumni and Advancement Center, Praxis began making the plain carbohydrate vaccine for toddlers in 25-microgram doses for shipment across the country.
The income from those sales funded continued research, particularly the work on a vaccine that would be effective in infants. The breakthrough came when the team combined the plain carbohydrate molecule with a protein from a different bacterium. The result was an antigen that boosted immunity in children younger than two.
“The pediatric infectious diseases chief, Keith Powell, used the vaccine in his infant daughter, whose name is Lindsey, and he took the blood samples,” Anderson says. “With that set of before-and-after blood samples, the rise in antibody was so dramatic that I knew it was going to work.”
Anderson later endowed a professorship in recognition of Lindsey Powell, the Lindsey Distinguished Professorship for Pediatric Research, a position currently held by Francis Gigliotti.
Another physician, Michael Pichichero, who was on the faculty at the time but is now in private practice, also was instrumental in testing the early iterations of the vaccine. “And that sort of helped refine the chemistry of it,” Anderson says, “to optimize how to put the vaccine together.”
By the time that Praxis demonstrated the effectiveness of the technology, commercial companies had made considerable progress on their own versions of a Hib conjugate vaccine. “All of a sudden, there were three or four Hib conjugates,” Anderson says, including versions that induced immunity against diphtheria, tetanus, pertussis, and hepatitis. Praxis was in danger of being left behind, but the team members turned their attention to whether the technology would work as a way to combat Streptococcus pneumoniae, a family of bacteria that also causes meningitis and other invasive diseases. A bacterium from that family was, at the time, the cause of millions of ear infections annually in children. Led by chemist Ronald Eby, the Praxis lab developed a pneumococcal conjugate, leading to vaccine technology that would be licensed to Wyeth Pharmaceuticals, now a subsidiary of Pfizer, and that would be marketed as the Prevnar vaccine. Members of the Wyeth team, including Dace Viceps Madore ’69, Maya Koster ’83, Eby, and Veluplillai Puvaneserajah, received the National Medal of Technology from President George W. Bush in 2007 for their work to develop Prevnar.
For his pioneering work, Anderson—along with Smith, who died in 1999, and Schneerson and Robbins—received the 1996 Albert Lasker Clinical Medical Research Award, one of the highest honors in medical science. Anderson was inducted into the National Academy of Sciences in 2010 and was appointed as a fellow of the American Academy of Microbiology in 2011. After he retired from the University in 1994, Anderson came out of retirement in 1996 to work on a new vaccine project with colleagues at Boston Children’s Hospital, where he and Smith first met nearly 30 years earlier. The goal is to develop an inexpensive vaccine against pneumococcus that can be applied nasally or orally in children, a delivery technique that would be cheaper and easier to implement in parts of the developing world.
“Even before [the Praxis technology] was licensed, it was understood that it would be so expensive that it couldn’t be afforded in the Third World, where the burden of pneumococcal disease is highest,” Anderson says. “People in the World Health Organization realized this and were wondering about what to do. I was part of the thinking about that, and I envisioned a very cheap way to make a vaccine that would prevent all the types of pneumococcal infection.
“I’ve been at that ever since. My motivation was that I knew the pneumococcal conjugate vaccine called Prevnar was going to be a financial success and a partial success for pneumococcal disease in the industrial world. And I thought, ‘Well, just for fun, I’ll try and do something that’s kind of a competitor of that,’ and so I did.”
Schor says she only learned the story of Rochester’s conjugate vaccine technology after taking the position as chair of pediatrics in 2006. The more she learned, the more she realized that the work represented an early example of what today is known as translational medicine—the idea that scientists can move a discovery into a viable, effective treatment more quickly when they are organized to bring their talents together.
“Now more than ever, the dialogue between the bench and the clinic is critically important,” she says. The academic background, training, and skills of several perspectives can be a powerful combination, especially when brought together as a team.
“The power really is in the partnership between the scientist and the clinician.”