Features
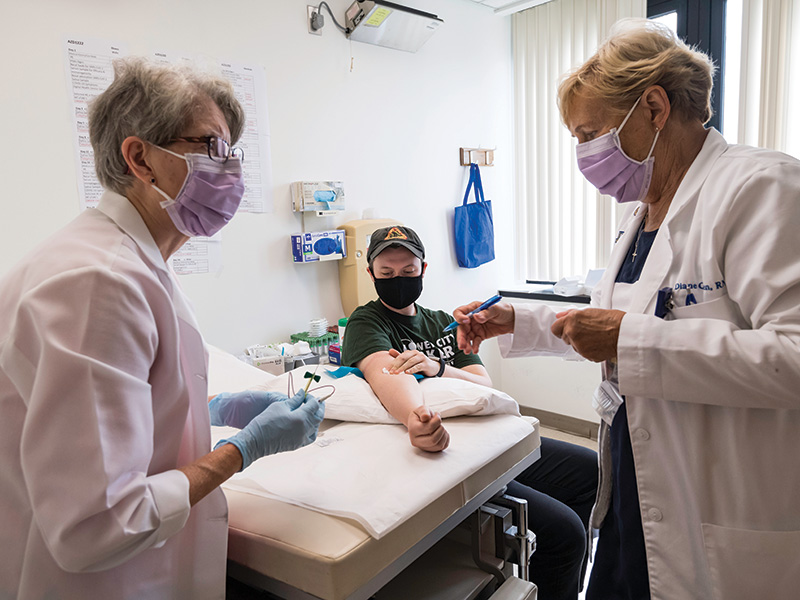 TESTING SITE: Licensed practical nurse Linda Anderson (left) works with
clinical research coordinator Doreen Francis to prepare Eron Damercy of
Rochester for a COVID-19 vaccine trial at the Medical Center. (Photograph by J. Adam Fenster)
TESTING SITE: Licensed practical nurse Linda Anderson (left) works with
clinical research coordinator Doreen Francis to prepare Eron Damercy of
Rochester for a COVID-19 vaccine trial at the Medical Center. (Photograph by J. Adam Fenster)
Last January, before Zoom meetings were the norm and travel restrictions were instated as part of lockdowns across the country, members of the University of Rochester’s Vaccine Trial and Evaluation Unit (VTEU) traveled to the National Institutes of Health (NIH) headquarters in Maryland. The NIH was holding its annual inaugural meeting of representatives from the nine organizations in the US serving as VTEU sites.
The meeting was led by the director of the National Institute of Allergy and Infectious Diseases, Anthony Fauci.
“Dr. Fauci comes into the room and he says, ‘Have you heard of this coronavirus coming out of China?’ ” says Angela Branche, an assistant professor of medicine and codirector of Rochester’s VTEU.
As most of the people in the room were respiratory scientists, they had indeed heard about SARS-CoV-2, the novel strain of virus that causes COVID-19.
The virus had begun making its way across the globe. At the time, however, information about the pathogen was extremely limited.
“That was actually the day we had our first case here on US soil, so it was just kind of a remarkable moment in history to be given our marching orders on the day when COVID-19 actually became a real problem in the United States,” Branche says.
Fauci told the group that combating the novel coronavirus would be their first mission as a new cohort of VTEU sites. With their directive clear, Rochester’s teams began mobilizing to start testing treatments and vaccines for COVID-19.
 COVID CHALLENGE: “We work seven days a week,” says Angela Branche (left), who with Ann Falsey, codirects Rochester’s VTEU. (Photograph by J. Adam Fenster)
COVID CHALLENGE: “We work seven days a week,” says Angela Branche (left), who with Ann Falsey, codirects Rochester’s VTEU. (Photograph by J. Adam Fenster)
More about Rochester’s leadership in vaccine development.
Rochester’s innovative Hib vaccine transformed pediatric medicine over the past quarter century.
The 2005 announcement of a vaccine against cervical cancer was a breakthrough chapter in a Rochester-born effort begun two decades earlier.
The team that used Rochester’s Hib vaccine technology to develop the groundbreaking Prevnar vaccine received National Medals of Technology.
‘A Therapeutic Wrench’
Rochester scientists leverage research on RNA to fight COVID-19.
Viruses like the coronavirus that causes COVID-19 are able to unleash their fury because of a devious weapon: ribonucleic acid, also known as RNA. COVID-19, short for “coronavirus disease 2019,” is caused by the novel coronavirus SARS-CoV-2. Like many other viruses, SARS-CoV-2 is an RNA virus.
This means that, unlike in humans and other mammals, the genetic material for SARS-CoV-2 is encoded in ribonucleic acid. The viral RNA is sneaky: its features cause the protein synthesis machinery in humans to mistake it for RNA produced by our own DNA.
A contingent of researchers at Rochester study the RNA of viruses to better understand how RNAs work and how they are involved in diseases. As COVID-19 continues to spread around the globe, the research provides an important foundation for developing antiviral drugs, vaccines, and other therapeutics to disrupt the virus and stop infections.
“Understanding RNA structure and function helps us understand how to throw a therapeutic wrench into what the COVID-19 RNA does&#mdash;make new virus that can infect more of our cells and also the cells of other human beings,” says Lynne Maquat, the J. Lowell Orbison Distinguished Service Alumni Professor in biochemistry and biophysics at Rochester and the director of Rochester’s Center for RNA Biology.
In the past few decades, as scientists came to realize that genetic material is largely regulated by the RNA it encodes, that most of our DNA produces RNA, and that RNA is not only a target but also a tool for disease therapies, “the RNA research world has exploded,” Maquat says. “The University of Rochester understood this.”
In 2007, Maquat founded the Center for RNA Biology as a means of conducting interdisciplinary research in the function, structure, and processing of RNAs.
The center involves researchers from Arts, Sciences & Engineering on the River Campus and at the Medical Center, combining expertise in biology, chemistry, engineering, neurology, and pharmacology.
As lab spaces began to reopen on campus this summer, many researchers pivoted to coronavirus research.
Maquat’s research focuses on nonsense-mediated mRNA decay (NMD), a mechanism that our cells use to combat viruses. Recently, she began testing how viral proteins, such as those involved in COVID-19 infections, can inhibit the mechanism’s machinery.
“At this point, combating this pandemic is an ‘all-hands-on-deck’ situation,” says Elaine Sia, professor and former chair of biology.
In the Department of Biology, Dragony Fu, an assistant professor of biology, and Jack Werren, the Nathaniel and Helen Wisch Professor of Biology, received expedited funding awards from the National Science Foundation to apply their expertise in cellular and evolutionary biology to research proteins involved in infections from COVID-19.
The funding was part of the NSF’s Rapid Response Research (RAPID) program to mobilize funding for high priority projects.
Werren’s research will be important in ameliorating some of the potential side effects of COVID-19 infections, including blood clots and heart diseases, while Fu’s research will provide insight into the potential effects of viral infection on a host cell’s cellular processes.
“Identifying which cell functions are affected by the virus could help lessen some of the negative effects caused by COVID-19,” Fu says.
This research on the fundamentals of cellular processes highlights collaboration between the Medical Center and the River Campus.
“Our strength as a university is our diversity of research expertise, combined with our highly collaborative nature,” Fu says. “We are surrounded by outstanding researchers who enhance our understanding of RNA biology, and a medical center that provides a translational aspect where the knowledge gained from RNA biology can be applied for therapeutics.”
&#mdash;Lindsey Valich
Rochester’s VTEU
Directed by Branche and Ann Falsey, a professor of infectious diseases, the VTEU is one of two NIH networks at Rochester. The other is an HIV Vaccine Trials Network, run by Michael Keefer, also a professor of infectious diseases.
Both are part of a larger network called the Coronavirus Prevention Network, created this year to help combat COVID-19.
With a mission to develop vaccines and treatments for emerging pathogens that spans six decades, the VTEU program is the NIH’s oldest network. While Rochester had been a member of the VTEU for part of that history, in December 2019, the University was awarded one of only nine VTEU sites in the United States.
Only a week later, the Chinese government announced a newly discovered pathogen with pandemic potential.
“[We received accreditation] just in time for us to get involved in the national effort to develop vaccines for COVID-19,” Branche says.
As the COVID-19 pandemic hit the US and the race for developing, testing, and evaluating potential vaccines against COVID-19 intensified, pharmaceutical companies, federal agencies, and vaccine evaluation teams turned to Rochester.
In addition to its designation as a VTEU site and other important research, the University is home to the vaccine that all but eradicated Haemophilus influenzae type b (Hib) and played key roles in the vaccine against cervical cancer caused by human papillomavirus (HPV).
Some of the leading candidates for COVID-19 vaccines trace their roots to technology developed at Rochester, and members of the research community have played vital roles in studying the processes involved in the disease.
“The history of Rochester’s infectious disease unit is very strongly associated with respiratory viruses, so it’s a very natural place for this kind of work to get done,” says Edward Walsh, professor of infectious disease medicine, who has been involved in the University’s past and present vaccine development efforts. “There has been a lot of collective expertise from the people who’ve been through here.”
Operation Warp Speed
Under the umbrella of the VTEU, the Medical Center is testing several vaccines as part of Operation Warp Speed, a federal initiative that aims to deliver 300 million doses of a safe, effective vaccine for COVID-19 by 2021.
The goal of the effort is to bring together individual networks to expand the nation’s capacity to address the challenge posed by the pandemic.
“There are six vaccines that have been selected by Operation Warp Speed to move through the process,” Branche says. “We are involved in four of them, which is pretty remarkable for any single institution.”
In May, as part of a collaboration with Rochester Regional Health, the Medical Center was one of four sites in the US involved in pharmaceutical giant Pfizer’s phase 1 study for a COVID-19 vaccine developed by Pfizer and the German biotechnology company BioNTech. Although Pfizer did not join Operation Warp Speed and did not take federal money to pay for research and development for the vaccine, the company contracted with the US government to provide doses of the vaccine to Americans, contingent on the vaccine’s effectiveness.
Several months later, Rochester made national headlines when the Medical Center was the site where the first volunteer in the US received a dose of the Pfizer/BioNTech vaccine during phase 3 of the study. Phase 3 marks the final stage of a vaccine’s development before FDA approval, mass production, and distribution.
As of mid-November, the Pfizer/BioNTech vaccine was pending review by the FDA for Emergency Use Authorization after findings of the phase 3 trial showed it was 95 percent effective.
“The University of Rochester was one of only two sites in the world given the task of ‘First in Human’ studies of the mRNA vaccine construct that is now in phase 3 development and played a major role in making this possible,” says William Gruber, senior vice president for vaccine clinical research and development at Pfizer. “The Rochester group is a leader in guiding trial design, assuring safety of participants in the trials, and assuring acquisition of data that would help progress a potentially safe and effective vaccine.”
Another vaccine being tested at Rochester has been developed by British pharmaceutical company AstraZeneca and the University of Oxford. Falsey is a national coordinating investigator for the vaccine trial, which also traces its roots to Rochester, through a former faculty member named Tom Evans.
Evans, an associate professor in the division of infectious diseases from 1995 to 2000, is the CEO/CSO of Vaccitech, a University of Oxford start-up that worked on the early-stage development of the COVID-19 vaccine before AstraZeneca took over. He was part of the broader research community in infectious diseases that developed the HPV vaccine, although his focus was more on HIV during his time at Rochester. Evans has since returned to the Rochester faculty and today is an adjunct professor at the Medical Center, where he continues his vaccine-related research.
Research Roundup
Rochester scientists and clinicians have been at the forefront of research efforts focused on the COVID-19 pandemic. Here’s a brief roundup.
Is Remdesivir Effective?
Last spring, Rochester began participating in a clinical trial sponsored by the National Institutes of Health to evaluate the safety and efficacy of the antiviral drug remdesivir. The double-blind, placebo-controlled trial is led by Ann Falsey and Angela Branche, directors of Rochester’s Vaccine Trial and Evaluation Unit. The drug received emergency use authorization in May, and research has turned to studying remdesivir in combination with other anti-inflammatory drugs such as baricitinib and steroids.
Remdesivir, developed by Gilead Sciences, is a broad-spectrum antiviral treatment that has been previously tested in humans with the Ebola virus and has shown promise in animal models for treating Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), which are caused by other coronaviruses. Researchers believe the drug blocks RNA polymerase, an enzyme that is required for viral replication.
“The results with remdesivir are positive, but it’s not a miracle drug,” Falsey says. “It becomes our building block on which we try to improve.”
Does Convalescent Plasma Help?
In July, the Medical Center joined researchers in a national trial led by John Hopkins University to test whether transfusions of plasma from recovered COVID-19 patients can prevent infection in healthy individuals or speed recovery in people with mild COVID-19 infections. Rochester’s trial is led by nephrologist Martin Zand, professor of medicine and senior associate dean for clinical research at the Medical Center.
Convalescent plasma from recovered COVID-19 patients contains antibodies that flag the SARS-CoV-2 virus for destruction by the immune system. Researchers hope the antibodies against the virus will prevent healthy people from getting infected and will boost immune responses in volunteers with mild disease—keeping them out of the intensive care unit and helping them recover faster.
“Academic medical centers, and especially the University of Rochester, have the entire spectrum of development and testing built into what they do every day,” Zand says. “We have basic researchers who study how the virus infects cells; we have biosafety facilities to work with the viruses within human cells and in animal models; we have the experience of clinical trials. And this is all under one roof.”
Are Nursing Homes Safe?
A series of studies conducted by Rochester researchers indicates that older residents from underrepresented racial and ethnic backgrounds and their caregivers bear the severest burden from COVID-19 across the entire spectrum of US nursing homes and assisted living communities.
According to one study, conducted by Yue Li, a professor of public health sciences, nursing homes with disproportionately higher numbers of residents from underrepresented populations reported two to four times as many new COVID-19 cases and deaths per facility than other nursing homes. Li studied data reported from 15,587 nursing homes in the US.
Disparities of that magnitude, Li says, suggest that long-standing, fundamental inequalities in nursing homes resulting from segregated facilities with limited resources and poorest quality of care are being “exacerbated by the pandemic.”
Another study, conducted by Helena Temkin-Greener, a professor of public health sciences, and using data from 4,685 assisted living communities in the US, found a fourfold higher COVID-19 case fatality rate among US assisted living communities, compared to the counties in which the facilities are located.
How Do Immune Systems Respond?
Rochester infectious disease experts have launched a study to understand how the body’s immune system responds to COVID-19 infection, including if and when a person could be reinfected with the virus and whether some people have preexisting immunity.
The researchers, including Dave Topham, the Marie Curran Wilson and Joseph Chamberlain Wilson professor of microbiology and immunology and an investigator of the Medical Center’s VTEU, are studying blood samples from people who recovered from mild to moderate COVID-19 as compared to blood samples from healthy donors whose samples were collected 6 to 10 years ago, long before they could have been exposed to COVID-19.
The team’s first published paper suggests that the seasonal colds people have had in the past may provide some protection from the virus and that immunity to COVID-19 is likely to last a long time—maybe even a lifetime.
—Lindsey Valich
A Robust History
When Branche first came to the University eight years ago as a fellow in infectious diseases, she didn’t plan on staying.
“I came here, and I thought I would do my two years of infectious disease training and then go back to New Jersey and set up a practice and that’ll be that,” Branche says. “Of course, once you come to Rochester, it’s like quicksand; you never leave, which is good. I’m just one of a long line of people who came to Rochester to train and realized that there’s more to medicine than just the practice of it; there’s what’s happening behind the scenes to invent and innovate and make medicine better.”
The institution’s strong lineage in the fields of vaccine technology and infectious disease research, combined with an institutional ethos of collaboration among clinicians and basic science researchers, has brought Rochester to the forefront of the medical community in the face of a global pandemic.
Rochester’s history of infectious disease research dates back to the late 1960s, when the University hired Gordon Douglas from the NIH as chief of the newly created department of infectious diseases. Under Douglas’s direction, the department brought in new faculty members committed to studying infectious diseases such as seasonal flu and viruses including human immunodeficiency virus (HIV) and respiratory syncytial virus (RSV).
In the 1980s, Rochester entered the “golden age” of vaccine research. That was when a Medical Center team led by pediatrician David Smith and including chemist Porter Anderson and pediatric immunologist Richard Insel ushered in a new era of pediatric medicine with the development of an innovative approach to boosting the immunity-inducing power of vaccines. Their technology was a key component in the development of the vaccine that eradicated Hib, a bacterium that typically attacks children younger than five and can cause serious illnesses such as meningitis and pneumonia.
Throughout the 1980s, the team worked through iterations of the vaccine. When Smith found he couldn’t interest a pharmaceutical company in commercializing their research, the three established their own start-up, Praxis Biologics.
In 1982, Walsh—and later Falsey—began collaborating with Smith and Praxis on developing a vaccine against RSV.
Meanwhile, in another nondescript room at the Medical Center, two Rochester professors of medicine, Richard Reichman and William Bonnez, and then graduate student Bob Rose, were developing technology that would prove to be instrumental in vaccines against HPV, a family of sexually transmitted viruses associated with diseases of the reproductive system, including cervical cancer.
Such collaborations between clinicians and researchers studying fundamental science principles are a hallmark of Rochester that make it a hub for revolutionary discoveries, says Stephen Dewhurst, chair and the Albert and Phyllis Ritterson professor of microbiology and immunology and vice dean for research in the School of Medicine and Dentistry.
“The University of Rochester has always been a very collaborative place, with partnerships involving basic science and clinical/translational research,” Dewhurst says. “This has been a big driver of Rochester being a site for all of this groundbreaking research in infectious diseases and vaccines.”
Pfizer Comes Calling
A vaccine against RSV has yet to be developed, but one of Walsh’s most significant contributions to RSV research was studying the proteins involved in RSV that allowed the virus to attack human cells.
“I had purified one of the important proteins involved in RSV, called a fusion protein,” Walsh says. “Praxis wanted to develop a vaccine for RSV, so we would help them in many aspects of this research.”
Praxis eventually became so successful in their research efforts that they were bought out by Pfizer, and many of the people—scientists and administrators—that Walsh and Falsey had worked with over the years in developing an RSV vaccine ended up at Pfizer.
Walsh and Falsey continued to work with Pfizer on RSV–related research, also studying seasonal coronaviruses—the family of viruses that now includes the pandemic SARS-CoV-2 coronavirus.
Through research conducted at Rochester and technologies originally developed by Walsh, Smith, and the Praxis group at Rochester for RSV, NIH investigators gained critical knowledge that is now being applied to a vaccine for COVID-19: specifically, fixing the fusion protein in the right shape and stabilizing it, which is key in developing a vaccine that will prevent SARS-CoV-2 from attaching to healthy cells.
When it became clear in the spring that a new strain of coronavirus was the cause of an escalating pandemic, Pfizer turned to Rochester to help evaluate its vaccine efforts, drawn by the historical relationships that had been built between researchers at the pharmaceutical company and the University.
“Ed Walsh and Ann Falsey are recognized scientific and clinical trial experts in understanding the epidemiology of respiratory diseases and conducting clinical trials of intervention strategies, including vaccines,” Gruber says. “And Ann directs an internationally recognized NIH–sponsored vaccine evaluation unit that provides expertise and infrastructure for clinical trials of vaccines. It was therefore natural to call upon this expertise for investigation of a vaccine against COVID-19.”
Just as it had in the 1980s and ’90s, Rochester again became an epicenter for developing treatments and vaccines.
An ‘Incredibly Intense’ Experience
With COVID-19 still raging across the globe, researchers and clinicians at Rochester and around the world continue to work tirelessly to develop vaccines and treatments to fight the pandemic.
“It’s been incredibly intense,” Branche says. “It’s exciting on the one hand, because we’re on the forefront of things that are happening very quickly. But it’s very tiring. We work seven days a week. We eat, breathe, and sleep COVID.”
However, she says, as infectious disease doctors, “this is what we train for.“It’s good to be a part of it and to be able to have an impact. It’s good to know that we’re contributing to the science that’s really needed right now.”

