|
|
|
|
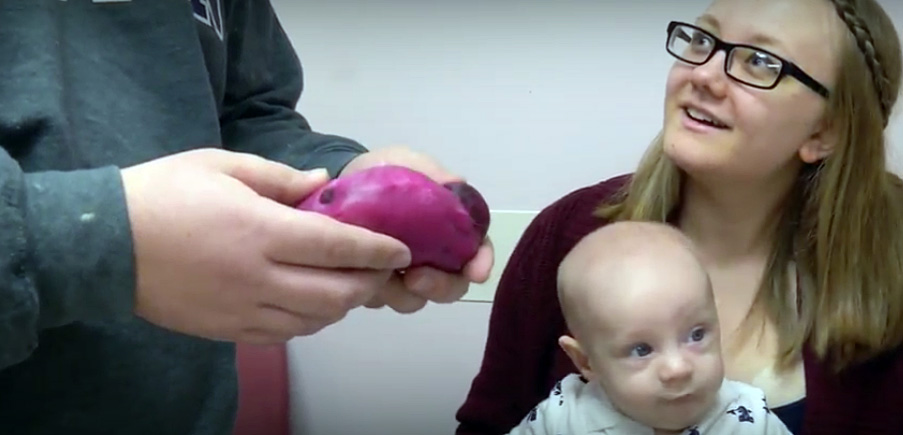 Screen grab from video shows Anthony Camnetar holding an exact replica of his kidney – complete with tumors –while his wife and son look on. The replica enabled Medical Center urologist Ahmed Ghazi to practice the complex surgery Anthony required long before he went under the knife.
Fake organs guide way for 'impossible' kidney surgeryAnthony Camnetar has undergone multiple surgeries to remove tumors that proliferate in his organs because of von Hippel-Lindau syndrome, a rare genetic disorder.
But the most challenging of all was the surgery he needed to remove five tumors from his left kidney.
Kidneys are highly prone to bleeding. Despite their small size, they receive 20 percent of the heart’s blood output. So surgeons will typically clamp the vessel that supplies blood to the organ before resecting the tumors. However, this makes the procedure a race against the clock as blood flow must be restored within 30 minutes or the kidneys become irreversibly damaged.
Fortunately, Anthony reached out to Ahmed Ghazi, an assistant professor in the Medical Center’s Department of Urology and the Wilmot Cancer Institute. Because of the complications from a surgery on his other kidney, Anthony wanted to explore the possibility of having the next procedure done robotically and had heard about Ghazi’s unique approach.
Ghazi partnered with Jonathon Stone, then a resident in the URMC Department of Neurosurgery, to establish the Simulation Innovation Laboratory. The lab fabricates lifelike artificial organs, which allow surgeons to practice complex cases in advance of the actual surgery.
The process begins by converting MRI, CT, or ultrasound scans obtained from the patient into computer-assisted designs (CAD). The CADs of organs are converted into molds, or negatives, which are built using a 3D printer. In a process akin to casting a bronze statue, the molds are then injected with a hydrogel which, after freezing, assumes a solid state.
By altering the concentration of the hydrogel, the team can add denser tumor mass and other components of the organ, such as blood vessels. The team also assembles entire segments of the body, complete with artificial muscle tissue, skin, and fat, and, depending upon the area of interest, the liver, intestines, spleen, kidney, and other adjacent organs and structures. Artificial blood vessels are connected to bags of red dye that will “bleed” if cut. This process allows surgeons to replicate the entire surgical process of guiding instruments to the right location, moving other organs out of the way, clamping blood vessels, and resecting and removing tumors.
“These rehearsals allow you to save operating time, decrease blood loss, potentially avoid complications, and prevent time you have to trouble shoot,” said Ghazi. “All of this directly impacts patient outcomes.”
Months ahead of the planned surgery, Ghazi created five replicas of Anthony’s kidney and conducted full rehearsals in the operating room using the robots, including a final practice run the day before the actual surgery.
“I started rehearsing using different game plans and deciding which tumors I was going to remove first,” said Ghazi. “And you can only figure that out by trying it a couple of times. It turns out that two of the five tumors could be removed before I cut off the blood supply and the rest needed to be done after the blood vessel was clamped.”
When the day came for Anthony’s surgery, Ghazi was confident he had developed the right approach. He successfully removed all five tumors, preserved 90 percent of the kidney’s function, and was able to restore blood flow in under 24 minutes.
“Recovery was so easy,” said Anthony. “A week after surgery I was able to walk around. The last surgery I had it took about a month and a half for me to be able to walk around without pain. I was able to hold my son two and a half weeks this time as opposed to two and a half months.”
Read more and see a video here.
Uncertainty in a date dampens interest in a mateAccording to a new study, those who feel greater certainty that a prospective romantic partner reciprocates their interest will put more effort into seeing that person again, while rating the possible date as more sexually attractive than they would if they were less certain about the prospective date’s romantic intentions.
Published in Computers in Human Behavior, the study by researchers from Israeli-based Interdisciplinary Center Herzliya and the University of Rochester finds that uncertainty about potential partners’ romantic interest decreased their sexual appeal.
“People may protect themselves from the possibility of a painful rejection by distancing themselves from potentially rejecting partners,” explains study co-author Harry Reis, a professor of psychology and Dean’s Professor in Arts, Sciences & Engineering at Rochester.
While some scientists have argued that uncertainty spices up sexual desire, Reis says his team’s results suggest the opposite holds true. “People experience higher levels of sexual desire when they feel confident about a partner’s interest and acceptance,” says Reis.
Lead author Gurit Birnbaum, a social psychologist and associate professor of psychology at the IDC Herzliya, says the findings suggest that sexual desire may “serve as a gut-feeling indicator of mate suitability that motivates people to pursue romantic relationships with a reliable and valuable partner.” Conversely, “inhibiting desire may serve as a mechanism aimed at protecting the self from investing in a relationship in which the future is uncertain.”
Read more here.
PI oversight: Protecting privacy of subjects and confidentiality of data(This is part of a monthly series to help principal investigators understand their role in ensuring that human subject protection requirements are met in their studies.)
Department of Health and Human Service and Food and Drug Administration regulations (45 CFR 46; 21 CFR 56) define specific criteria that human subject research must meet in order for an Institutional Review Board (IRB) to approve the research. A critical, yet often overlooked step in the protocol development process is to objectively evaluate study protocols against these criteria prior to IRB submission. Doing so will help facilitate IRB review of the proposal, with the intent of minimizing IRB stipulations.
Over the upcoming months, as part the “PI Oversight Tip of the Month” series, each criterion for IRB approval will be reviewed. This month we look at approval criteria #7: “When appropriate, there are provisions to protect the privacy of subjects and maintain the confidentiality of the data.”
First it is important to understand the difference between privacy and confidentiality:
Privacy is defined as having control over the extent, timing, and circumstances of sharing oneself (physically, behaviorally, or intellectually) with others or access to people or people’s information.
Confidentiality is the process or method for ensuring that information collected from a subject is protected.
In evaluating this criterion, the IRB will consider:
- Are all study procedures (including recruitment and enrollment) conducted in a manner that ensures the protection of subject privacy?
- Will institutional privacy and security standards (including HIPAA, if applicable) be adhered to?
- Is all of the information collected and/or accessed necessary to meet the aims of the research?
- How and where will the data be stored?
- Will data be shared with individuals outside of the immediate study team? If so, was this disclosed in the consent form and what measures are in place to protect the subject’s confidentiality?
- Will the data be coded? Will the data be de-identified at any point and if so, when? For additional information on coding vs. de-identification of data see the OHSP 2015 Q4 Newsletter.
- If sensitive information will be collected, are there additional protections (e.g., Certificate of Confidentiality) in place?
Stay tuned next month for criteria #8, “when some or all of the subjects are likely to be vulnerable to coercion or undue influence…additional safeguards have been included in the study to protect the rights and welfare of these subjects.” For previously highlighted criteria, see the 11/10/2017, 1/5/2018, 2/9/2018, 3/23/2018, 4/20/2018, and 5/18/2019 editions of Research Connections.
Genomic data analysis workshops planned in JulyGenomic Data Analysis Workshops in July focus on providing biomedical researchers a basic understanding of experimental design as it relates to RNA-Seq and a “toolkit” to perform some analyses on RNA-Seq data. The workshops—which will be held July 9 and 10, and July 16 and 17—are a collaborative initiative between the Genomics Research Center and the Department of Biostatistics and Computational Biology. Space is limited. Register by June 30.
Funding available for environmental health pilot projectsThe Medical Center’s Environmental Health Sciences Center (EHSC) is offering up to $30,000 for one year of funding for pilot projects relevant to the theme, “Environmental Agents as Modulators of Human Disease and Dysfunction.” Proposals that investigate how the environment modifies stem or progenitor cells, affects early origins of adult disease, or host-pathogen interactions are especially welcomed.
Applicants are encouraged to use emerging technologies (CRISPR, next generation or single cell RNA sequencing, big data initiatives, etc.) and the unique core facilities of the center. Applicants must hold a tenure-track faculty position, but new investigators collaborating with existing EHSC faculty are also encouraged to apply.
Submit initial applications to Pat Noonan-Sullivan by Wednesday, August 1, 2018.
Additional Information.
PhD dissertation defensesKelly McGlynn, pharmacology, “Cooperative and Mechanistic Roles of Mecom in Hematopoiesis,” 3 p.m. June 25, 2018. Case Method Room (1-9576), Medical Center. Advisor: Archibald Perkins.
Dev Crasta, clinical psychology, “The Promoting Awareness and Improving Relationships (PAIR) Program: Investigating the Efficacy and Mechanisms of a New Relationship Enhancement Program.” 10 a.m., June 28, 2018. Meliora 366. Advisor: Ronald Rogge.
Greg Madejski, biomedical engineering, “Silicon-Based Nanomembrane Uses in Biosensing.” 1 p.m., June 28, 2018. 1400 Wegmans Hall. Advisor: James McGrath.
Mark your calendarToday: Deadline to apply for pilot and feasibility awards of up to $50,000 for innovative applications of technology (e.g. novel use of electronic health record data, wearable sensors, digital tools, human-machine interfaces, etc.) in research with human participants to yield new insights into clinical neuroscience. The Center for Health + Technology (CHeT), in conjunction with the Ernest J. Del Monte Institute for Neuroscience. For more information and to download the RFA, click here.
June 27: “Identifying Differences in GPUs Using Performance Data.” Sreepathi Pai, assistant professor of computer science. Data Science Summer Colloquium Series, Goergen Institute for Data Science. Noon to 1 p.m., Wegmans Hall 1400. Open to all faculty, staff, students, and community members. Lunch included.
June 30: Deadline to register for Genomic Data Analysis Workshops, which will be held July 9 and 10 and July 16 and 17, to provide biomedical researchers a basic understanding of experimental design as it relates to RNA-Seq and a “toolkit” to perform some analyses on RNA-Seq data. A collaborative initiative between the Genomics Research Center and the Department of Biostatistics and Computational Biology.
July 11: “Information Flow in Music.” David Temperley, professor of music theory at the Eastman School. Data Science Summer Colloquium Series, Goergen Institute for Data Science. Noon to 1 p.m., Wegmans Hall 1400. Open to all faculty, staff, students, and community members. Lunch included.
July 17: CIRC Summer School begins. Classes in programming languages and data analysis skills. VISTA Collaboratory. Click here to learn more and to register.
July 18: “Physics of Complex Systems.” Gourab Ghoshal, assistant professor of physics. Data Science Summer Colloquium Series, Goergen Institute for Data Science. Noon to 1 p.m., Wegmans Hall 1400. Open to all faculty, staff, students, and community members. Lunch included.
Aug. 1: Deadline to submit initial applications for Environmental Health Sciences Center funding of up to $30,000 for pilot projects relevant to the theme, “Environmental Agents as Modulators of Human Disease and Dysfunction.” Submit initial applications to Pat Noonan-Sullivan. Additional Information.
|
|
|
|
|