|
|
|
|
 Graduate student and lead author Mingxiao Li holds a small lithium niobate chip in an etching chamber. (Photo courtesy of Qiang Lin’s lab)
A breakthrough in miniaturizing light-based chipsPhotonic integrated circuits that use light instead of electricity for computing and signal processing promise greater speed, increased bandwidth, and greater energy efficiency than traditional circuits using electricity.
But they’re not yet small enough to compete in computing and other applications where electric circuits continue to reign.
Electrical engineers at the University believe they’ve taken a major step in addressing the problem. Using a material widely adopted by photonics researchers, a Rochester team has created the smallest electro-optical modulator yet. The modulator is a key component of a photonics-based chip, controlling how light moves through its circuits.
In Nature Communications, the lab of Qiang Lin, professor of electrical and computer engineering, describes using a thin film of lithium niobate (LN) bonded on a silicon dioxide layer to create not only the smallest LN modulator yet, but also one that operates at high speed and is energy efficient.
This “paves a crucial foundation for realizing large-scale LN photonic integrated circuits that are of immense importance for broad applications in data communication, microwave photonics, and quantum photonics,” writes lead author Mingxiao Li, a graduate student in Lin’s lab. Read more here.
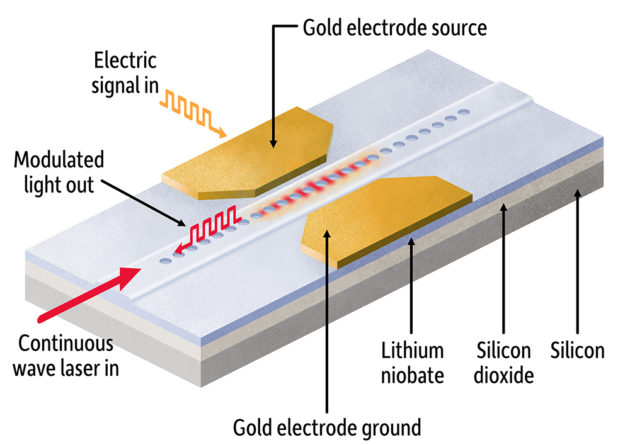
A schematic drawing shows an electro-optical modulator developed in the lab of Qiang Lin, professor of electrical and computer engineering. The smallest such component yet developed, it takes advantage of lithium niobate, a “workhorse” material used by researchers to create advanced photonics integrated circuits. (University of Rochester illustration / Michael Osadciw)
Medical Center joins phase 3 trial for potential coronavirus vaccineThe Medical Center is joining a phase 3 clinical trial for a potential coronavirus vaccine being developed by AstraZeneca and the University of Oxford known as AZD1222.
This is the second phase 3 coronavirus vaccine study to be conducted in Rochester. On July 27, four volunteers in Rochester were the first in the nation to receive an experimental vaccine being developed by Pfizer and BioNTech. Rochester was one of only four sites in the nation that also participated in early stage studies of the Pfizer/BioNTech vaccine. Phase 3 represents the final stage of human testing prior to regulatory approval, production, and mass distribution.
The Rochester arm of the AstraZeneca study is being led by Ann Falsey, Angela Branche, Mike Keefer, and Catherine Bunce. Falsey and Branche oversee the URMC Vaccine and Treatment Evaluation Unit and Keefer and Bunce lead the URMC HIV Vaccine Trials Unit. Both programs are part of the national COVID-19 Prevention Network (CoVPN), which was formed by the National Institute of Allergy and Infectious Diseases (NIAID) to help lead the scientific response to the pandemic.
The vaccine being developed by the British and Swedish company AstraZeneca and the University of Oxford uses a harmless adenovirus that contains the genetic material of the COVID-19 spike protein. The vaccine stimulates production of the surface spike protein, which primes the immune system to recognize the virus if infected.
Phase 1/2 studies conducted in the U.K. – the results of which were reported on July 20 in the journal Lancet – found that the vaccine was not only safe, but generated an immune response to the virus. Whether or not the vaccine provides protection from coronavirus infection across a wide range of age groups and medical conditions are questions that the phase 3 study will now seek to answer.
The randomized placebo-controlled clinical trial will recruit 30,000 people across 81 sites in the U.S. including 1,000 volunteers in Rochester. Researchers are focusing on individuals in the Rochester area ages 18 to 85 who are at greater risk for coronavirus infection, such as health care workers, first responders, teachers, and people who work in restaurants or retail. Because COVID-19 has had a disproportionate impact on people of color, researchers are working with community partners to invite Black and Latinx individuals to participate in vaccine trials.
Individuals interested in volunteering for the study can visit www.covidresearch.urmc.edu or call (585) 276-5212.
Why older people heal slower after injuring a muscleA new study published in Nature Communications provides insight into why older individuals take longer than younger people to heal following muscle injuries from a fall or exercise. Led by Joe V. Chakkalakal, Dean’s Associate Professor in the Department of Pharmacology and Physiology and Roméo Blanc, postdoctoral fellow in Chakkalakal’s lab, the study also highlights a potential path to speed muscle regeneration and strength recovery in older adults.
When any individual sustains a muscle injury there’s an inflammatory response. This response is prolonged in aged tissue and interferes with the ability of stem cells to heal the tissue. The research team discovered that a pathway known to be active in inflammatory cells – the CCR2 pathway – is also expressed in muscle progenitor cells. They found, unlike in young mice, persistent activation of CCR2 signaling in muscle progenitor cells in older mice after acute injury. Opportune inhibition of CCR2 post-injury enhanced aged regeneration and functional recovery in the mice.
“The CCR2 pathway is well known, but no one thought of looking for it or studying it in non-inflammatory cells,” said Chakkalakal, the corresponding author of the study. “We connected the entire pathway and found exactly how it is preventing the differentiation of progenitor cells into mature muscle in older animals.”
Next, the team plans to study how manipulating this pathway could supplement the beneficial effects of exercise on aged muscle. They are also interested in learning about the role this pathway plays in pediatric cancer survivors, who experience accelerated aging and muscle loss.
Read more here.
Congratulations to . . . Sydney Simpson, a graduate student in immunology, microbiology, and virology, and Omar Hedaya, a graduate student in biochemistry and molecular biology, who were top prize winners in an essay contest sponsored by the University’s Center for RNA Biology on “The Role of RNA research in community health.”
The $1,000 gold prize was awarded to Simpson’s “Nucleoside Analog Inhibitors: Timeless & Timely Beacons of Hope.” Hedaya’s “Know the Fundamentals when Seeking the Future” received the silver prize of $500. They will use their winnings toward technology needs for this semester.
Virtual symposium on future of clinical trials for Parkinson'sJoin the UR-Udall Center free Virtual Meeting + Symposium, hosted by the UR Center for Health + Technology (CHeT) on September 10 and 11 to discover the future of clinical trials for Parkinson’s.
Meet innovators from academia, industry, and advocacy; learn about virtual clinical trials, and connect with others during our interactive sessions. Register now.
IRB start-up packages for multi-site clinical trialsIf you are conducting a multi-site clinical trial that is funded by the NIH, let SMART IRB’s single IRB start-up packages steer you in the right direction.
Download the essential tools, templates, and checklists to help you prepare for and navigate sIRB arrangements for your studies, along with guidance on how, when, and why to use these resources.
Keeping abreast of the University's response to COVID-19
Here are important links for researchers:
Update on confirmed cases and future testing: With more than a month of testing students for coronavirus completed, the total number of positive cases stands at five. That’s out of more than 4,000 tests of undergraduate and graduate students who have arrived back on campus since August 1.
University Health Service will begin asking randomly selected students to take a COVID-19 test. The program is designed to monitor for potential outbreaks in the University community. Students selected for the surveillance testing program will be notified by email.
The Coronavirus Update website is updated when additional cases are confirmed or self-reported.
Update on policies for events, guests, and visitors: With the exception of guests and visitors who are considered essential to the University’s academic and research missions, Rochester’s campuses will continue to be closed to guests and visitors, including family members, going into the fall semester.
Part of updated guidance from the Coronavirus University Restart Team (CURT), the guidelines are intended to help limit the potential for the spread of coronavirus on campus and in surrounding communities.
The update also outlines policies for on-campus events and the process for requesting permission to hold an event.
Convalescent Plasma Gains Emergency Use Authorization: After a brief hold, the FDA authorized convalescent plasma therapy as an emergency treatment for COVID-19 on August 23. The emergency use authorization affirms that this treatment is likely safe and effective but only allows it to be used to treat hospitalized COVID-19 patients. There are still no approved therapies to prevent infection or treat mild cases of COVID-19. Currently, clinical trials, like those being run at URMC, are the only way for COVID-19 outpatients and healthy people who are at high risk for contracting COVID-19 to access convalescent plasma therapy.
Goergen Athletic Center reopens for students: The Goergen Athletic Center has reopened for use by full-time students only. The facility is not available for faculty, staff, or guest use until further notice. This decision is in line with the recent change in New York State guidelines permitting gyms to reopen. Strict occupancy limits are in place, requiring reservations for use of the Bloch Fitness Center. Students using the facility for recreational purposes are required to wear cloth masks (no bandanas, buffs, or gaiters) at all times. Regular hours are scheduled to start today.
Public Health Ambassador program—new for Fall 2020: Would you like to help educate the campus community on healthy behaviors, promote safe practices, and help ensure adherence to the new regulations during this pandemic? Check the UHS website to learn more about becoming a Public Health Ambassador. The program is open to all full-time undergraduate and graduate students. Interested students are asked to complete a brief application. If you have questions, email ldudman@uhs.rochester.edu.
Advice for helping children learn to wear masks: Elizabeth Murray, an assistant professor of clinical emergency medicine and of clinical pediatrics, shares ways to help children be safe and wear a mask at school.
More from Rochester’s medical experts: especially for those dealing with the stress of school and serious illness.
|
|
|
|
|