Challenging a long-held convention, University researchers have shown they can inhibit the influenza A virus by targeting its genomic RNA with “antisense” compounds.
Their findings, highlighted on the cover of Nucleic Acid Therapeutics, offer scientists a new way to attack an increasingly drug-resistant pathogen that causes an estimated 250,000 to 500,000 deaths a year.
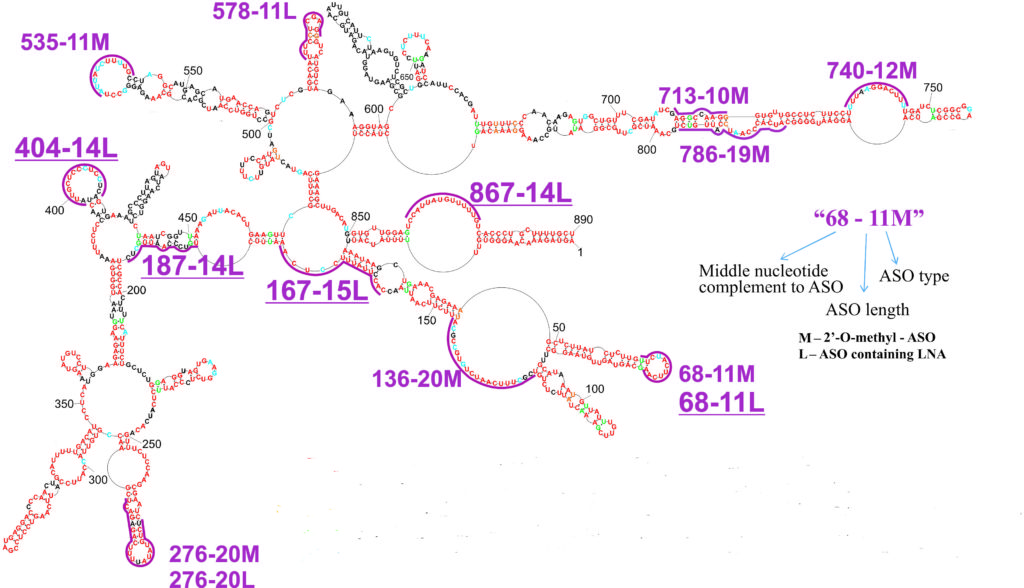
“Antisense” compounds are synthesized with nucleotides, the building blocks of nucleic acid, often shown as various combinations of A, U, G and C. When the compounds – called antisense oligonucleotides (ASOs) – bind to the targeted genomic RNA, they block its ability to replicate.
The collaboration, involving the labs of Douglas Turner, professor of chemistry; Luis Martinez-Sobrido, associate professor of microbiology and immunology; and two researchers in Poland, reported that “antisense” compounds targeting one of the virus’ eight genomic RNA segments caused a five- to 25-fold reduction of influenza A virus in cell cultures.
“That’s a big difference,” Martinez-Sobrido says. “When mice are infected with 10,000 viruses, they all die. However, with 25 times less virus, all animals can survive infection and they don’t even develop symptoms.”
The most effective of the antisense compounds ranged from 11 to 15 nucleotides long, and were not toxic to host cells.
To date, most “antisense” research has focused on targeting messenger RNA; the only two FDA approved “antisense” therapeutics – vitravene for use against a retina inflammation, and mipomersen to reduce cholesterol – do so.
“To my knowledge, this is the first published paper where ASOs target internal regions of genomic influenza viral RNA,” says Turner. “This genomic RNA has not been targeted because the dogma was that it is completely encapsulated by the viral nucleoprotein, and therefore is not accessible.”
But recent evidence has suggested – as this study demonstrates – that influenza genomic RNA is vulnerable, at least at certain points in the virus’ life cycle. For example, packaging of the influenza viral genome is “mediated by RNA-RNA interactions” among all eight segments, Martinez-Sobrido says. “So clearly during the replication cycle of influenza virus, the genomic RNA is at least partially naked.”
Influenza viruses have shown a remarkable ability to mutate and become resistant to current antiviral drugs.
Martinez-Sobrido believes ASOs could be more difficult for viruses to bypass. “If an oligonucleotide is targeting a segment of genomic viral RNA that is especially important, any mutation that altered that RNA would likely be lethal to the virus,” Martinez-Sobrido says. And if additional ASOs could simultaneously attack three or four other segments of genomic viral RNA, the virus’ task would be even more complicated.
The study was supported with funding from an NIH Fogarty International Research Collaboration Award received by Turner and Elzbieta Kierzek, associate professor at the Institute of Bioorganic Chemistry Polish Academy of Sciences. It was also supported by a 2014 University Research Award to Turner and Martinez-Sobrido. The team also included Prof. Ryszard Kierzak of the Polish Academy of Sciences, and University postdoctoral fellows Aitor Nogales in Martinez-Sobrido’s lab and Elzbieta Lenartowicz, the lead author, in Turner’s lab.
For their work in this area, Turner and Ryszard Kierzak have been named recipients of the Poland – U.S. Science Award this year from the American Association for the Advancement of Science (AAAS) and the Foundation for Polish Science.
Martinez-Sobrido and Turner are both members of the University’s Center for RNA Biology, which draws more than 20 faculty members from seven departments to conduct interdisciplinary research into the function, structure, and processing of RNA. The center is directed by Lynne Maquat, professor of biochemistry and biophysics, who is considered a pioneer of nonsense-mediated mRNA decay.