University of Rochester researchers are building technology to predict the course of tendon injuries in individual patients—a form of personalized medicine that will lead to more effective treatments.
Too often, promising therapeutic drug candidates that are developed as a result of expensive animal studies prove ineffective—or even dangerous—when tested in humans. Although lab animals may have similar anatomical features to humans, their physiology, metabolism, and genetic diversity can be quite different.
Three University of Rochester biomedical researchers are addressing the problem through a novel form of personalized medicine. They are developing an alternative “organ on a chip” technology that uses tissue samples from an individual human patient to mimic how a disease or disorder might occur in that patient—in this case, scarring from a tendon injury, especially after surgery to repair the damage.
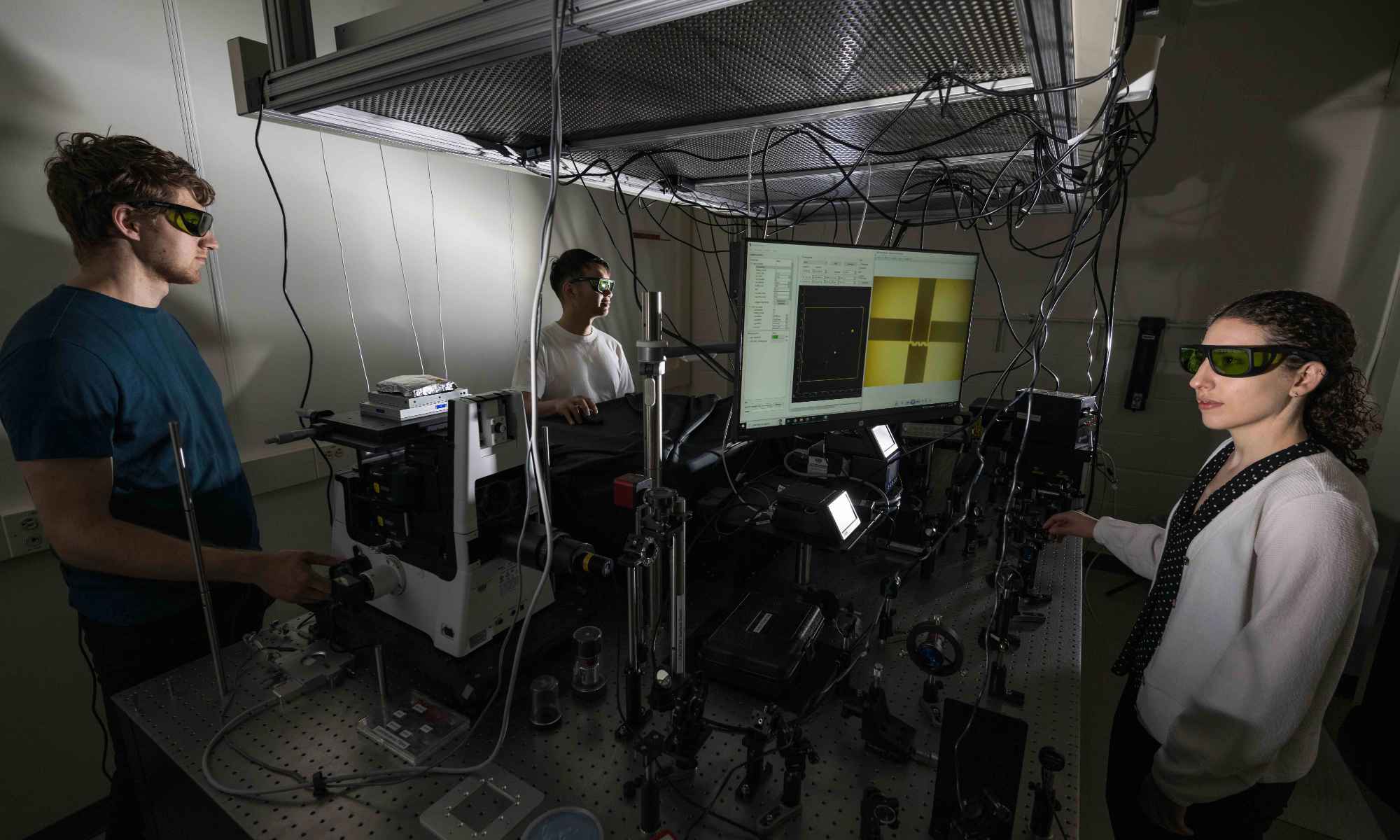
“This technology is likely the future of medicine. You can now think about personalized medicine in a chip,” says Hani Awad, the Donald and Mary Clark Distinguished Professor in Orthopaedics and professor of biomedical engineering at the Center for Musculoskeletal Research. He is collaborating on the project with James McGrath, professor of biomedical engineering, and Benjamin Miller, professor of dermatology, biomedical engineering, optics, and biochemistry and biophysics.
The collaboration—aided by the close proximity of the University of Rochester Medical Center and its Center for Musculoskeletal Research to the Department of Biomedical Engineering at the nearby River Campus—represents “an exquisite blend of biological science and engineering,” Awad says. “The team outside the three of us includes sensor scientists, orthopedic surgeons, and immunologists. It’s a very multidisciplinary approach.”
The research is supported by a $3.8 million grant—one of 10 nationwide—from the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), which is charged with improving and streamlining the processes by which new treatments and cures can be delivered to patients. The grants are administered through a new program, Clinical Trials on a Chip, which is led by NCATS in conjunction with several other NIH institutes and centers, including the National Cancer Institute, the National Institute of Child Health and Human Development, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. NCATS began funding research on tissue chips for drug screening in 2012.
A major goal of the program is to develop 3-D platforms engineered to support living human tissues and cells and mimic complex biological functions of organs and systems, which could better predict which patients are most likely to benefit from an investigational therapy prior to initiating clinical trials.
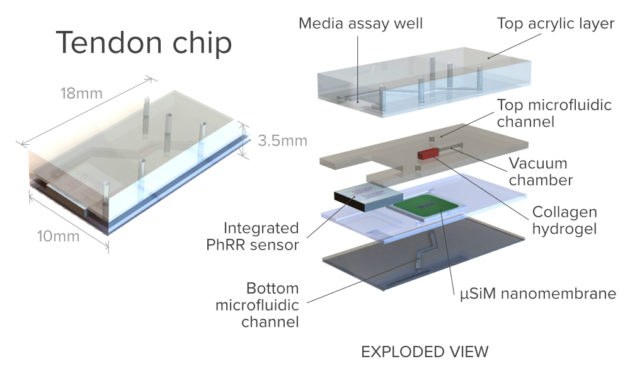
To the best of the researchers’ knowledge, the Rochester project will be the first organ-on-a-chip platform designed for modeling scar formation in tendons.
How tendons respond to injury
Awad, whose lab studies the response of musculoskeletal tissue to injury, has a specific interest in tendons.
When tendons are injured, they heal much like our skin, Awad says. They form a scar. “And that scar tissue, especially if surgery was involved in repairing the injury, often compromises the subsequent mechanical function of the tendon,” he adds. This can result in less strength or flexibility in a limb or joint, or even eventual reinjury or rupture.
Paradoxically, this scarring is the result of an inflammatory response “that nature designed to protect us from invading microbes at the site of the injury,” Awad explains. “We obviously have some bleeding, we have some new blood vessels forming, and you have infiltrating cells from the immune system coming to the site of the injury.” When this process is prolonged, fibrotic scar tissue is produced.
“So, the response to the injury is an interplay of tendon tissue, inflammatory cells, and the microvasculature (the system of tiny blood vessels that perfuse body tissue). And this is exactly what we would like to replicate in the chip,” Awad says.
Two new ‘twists’
And that’s where McGrath’s lab becomes involved. For several years his lab has been perfecting its patented ultrathin silicon nanomembranes—less than 200 nanometers thick—for a host of applications, including their use as “ideal barriers” in multi-compartment devices to culture and study human tissue.
So, in that sense, the multi-compartment, microfluidic tendon-on-chip platform his lab will create for this project “will definitely be based on things we’ve done in the past,” McGrath says. “We’ve been getting progressively better at it, and some of the advancements we’ve made in the last couple of years were a big help in securing this grant.”
However, this platform will take his research a step further. “It will need to incorporate mechanical action to extend the tendon on the other side of the blood compartment,” he says. And that “will certainly be groundbreaking in terms of the functionality that we bring to it.”
Thanks to Miller’s lab, the device will be groundbreaking in another way as well.
“Often with an organ on a chip, researchers allow the experiment to run for a while, and then they do an analysis using immunohistochemistry, which means you mix antibodies that have a florescent tag into the system, then look at it with a fluorescence microscope to see what the cells are doing. But once you do that, the system is dead,” says Miller, an expert in photonic biosensors. “Or, if you don’t want to kill the system, you have a sensor somewhere downstream, disconnected from the chip.”
“Our goal is to bring the sensors and chip together, so that in real time you’re getting a readout of whatever is happening in the chip, without interrupting [the system],” Miller says.
Based on work his lab has done as part of the AIM Photonics initiative, Miller’s lab will contribute a “very small format, planar sensor device,” incorporating photonic ring resonators and 2-D photonic crystals. Placed in close contact with McGrath’s organ on chip platform, the sensors will enable researchers to noninvasively measure proteins secreted as part of the inflammatory response.
“By the end of this grant, the goal is for us to be able to take cells from a patient, and actually incorporate them in multiple devices, so you can look at several potential treatments at once,” Miller says. “Let’s say that you had an injury and tendon repair, and the doctor wants to know what the best therapy is going to be—not generically, but what’s the best therapy for you. And that’s what we’ll be able to determine. That’s the really cool aspect of this.”