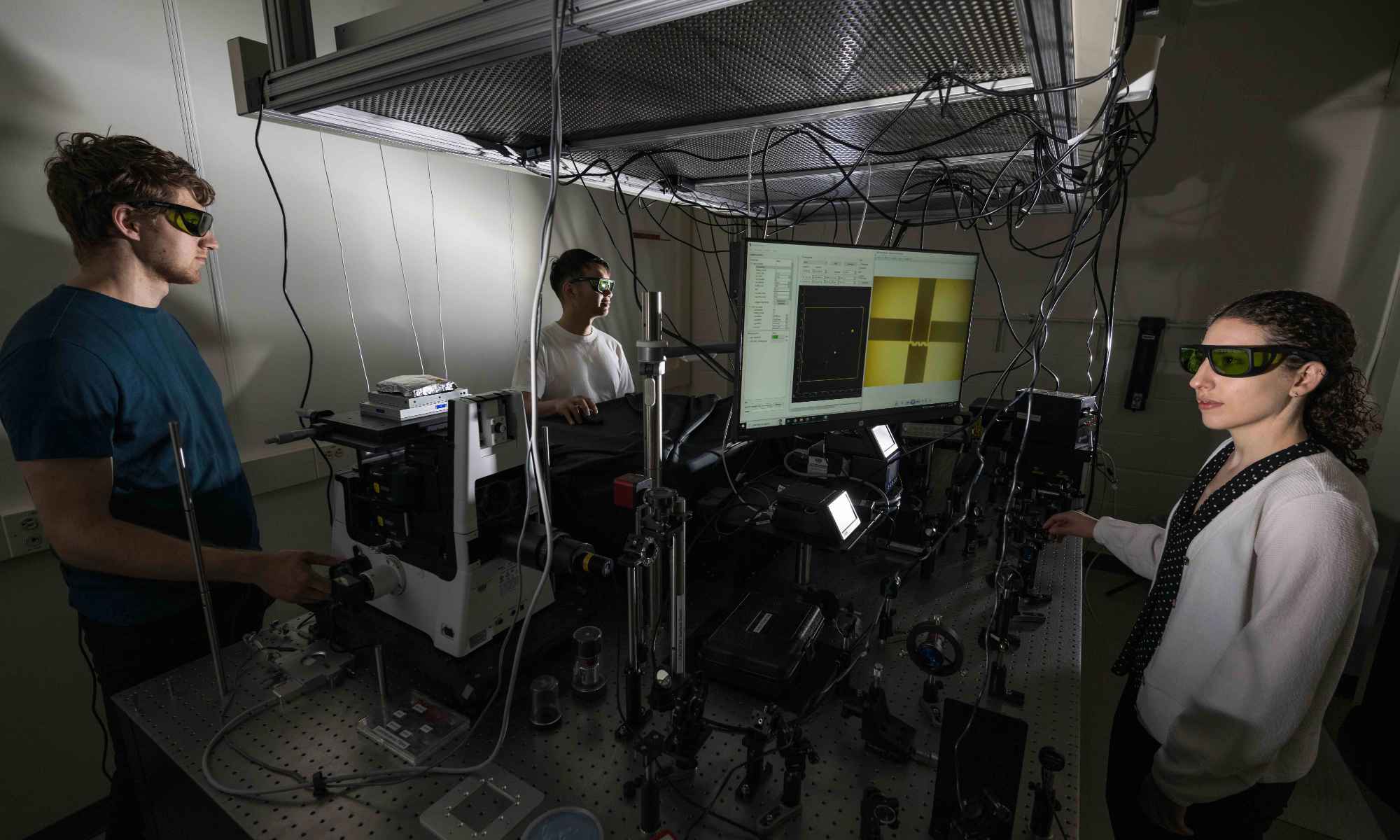
Rochester scientists have secured national funding for a multi-institutional research effort that could alter the basic rules of chemistry.
University of Rochester chemist Todd Krauss will lead a multi-institution effort to transform the field of chemistry, thanks to a $1.8 million dollar grant from the National Science Foundation (NSF). Chemists have long understood the tools they have available in order to create new molecules, such as changing the reaction temperature or using a catalyst—or doing both. Now, Krauss and his fellow researchers want to use light to facilitate previously impossible chemical reactions—in essence, by turning light into a catalyst.
“This is a high-risk proposal,” says Krauss, a professor of chemistry and of optics at Rochester. “Will we be able to get enough molecules to strongly interact with the light to make a difference? If we do, it could be a paradigm shift in the field of chemistry.”
Quantum principles applied to chemistry
According to the principles of quantum science—which deals with the fundamental nature of atoms and subatomic particles—light is made up of small, discrete packets of energy. Krauss’s project calls for putting molecules in an optical cavity and using those discrete packets to change the energy states of electrons in the molecule. When that’s done, the molecules behave differently, opening the door for new bonding possibilities and ,thus, new chemistry.
Molecules form through chemical bonding—the sharing of orbiting electrons. By changing temperatures or introducing catalysts, chemists can manipulate how—or whether—electrons are shared. These interactions follow basic rules. For example, carbon-chlorine bonds are broken more easily than carbon-hydrogen bonds. Here, the team aims to use the application of quantum principles in order to change these basic rules to allow different bonds to break and reform.
Krauss hopes to alter the spatial properties of electrons, and as a result, change the way molecules bond.
“We can potentially move electrons uphill from one molecule to another—something that has been classically forbidden,” says Krauss. “Most electrons have spherical orbits. If we can move some of those electrons into non-spherical orbits, they’ll behave differently. Doing that would allow us to create new molecules.”
QuEST for better medications, greener energy, new materials
According to Krauss, this work represents a new way of developing chemical reactions—one with many potential benefits to society. “In theory, that could lead to new applications in fuel production, pharmaceuticals, and the manufacturing of plastics,” he says.
The University of Rochester has a long tradition of quantum science with respect to strongly coupling light and atoms—defining the field of quantum optics for decades—dating back to the pioneering work of Leonard Mandel and Emil Wolf more than five decades ago. In QuEST, the team will build on that tradition by exploring how to strongly couple light with molecules in order to manipulate chemical reactions, pushing quantum optics into new and uncharted territory.
Under the terms of the grant, Krauss will direct the NSF Phase I Chemical Innovation Center for Quantum Electrodynamics for Selective Transformations (QuEST). The QuEST research team includes fellow University of Rochester chemistry professors Pengfei Huo and William Jones, as well as optics professor Nick Vamivakas. Joining them on are Jillian Dempsey at the University of North Carolina–Chapel Hill, Nicolas Large and Zachary Tonzetich from the University of Texas–San Antonio, Teri Odom from Northwestern University, and Daniel Weix from the University of Wisconsin–Madison.
“This is a three-year seed grant,” explains Krauss. “After a couple of years, we’ll compete for a Phase II grant—$20 million dollars over four years—to continue the research.”
Read more
 Chemists go ‘back to the future’ to untangle quantum dot mystery
Chemists go ‘back to the future’ to untangle quantum dot mystery
For more than 30 years, researchers have been creating quantum dots—nanoscale semiconductors with remarkable properties. But quantum dot synthesis has occurred largely by trial and error. Thanks to the work of two Rochester chemists, that may be about to change.
 Light-emitting quantum dots could ease synthesis of novel compounds
Light-emitting quantum dots could ease synthesis of novel compounds
Most chemists have studied quantum dots for their basic properties. But new research by Rochester scientists points to potential applications in the synthesis of pharmaceuticals, fine chemicals, and agro-chemicals.
 Building a quantum network one node at a time
Building a quantum network one node at a time
New research demonstrates a way to use quantum properties of light to transmit information, a key step on the path to the next generation of computing and communications systems.