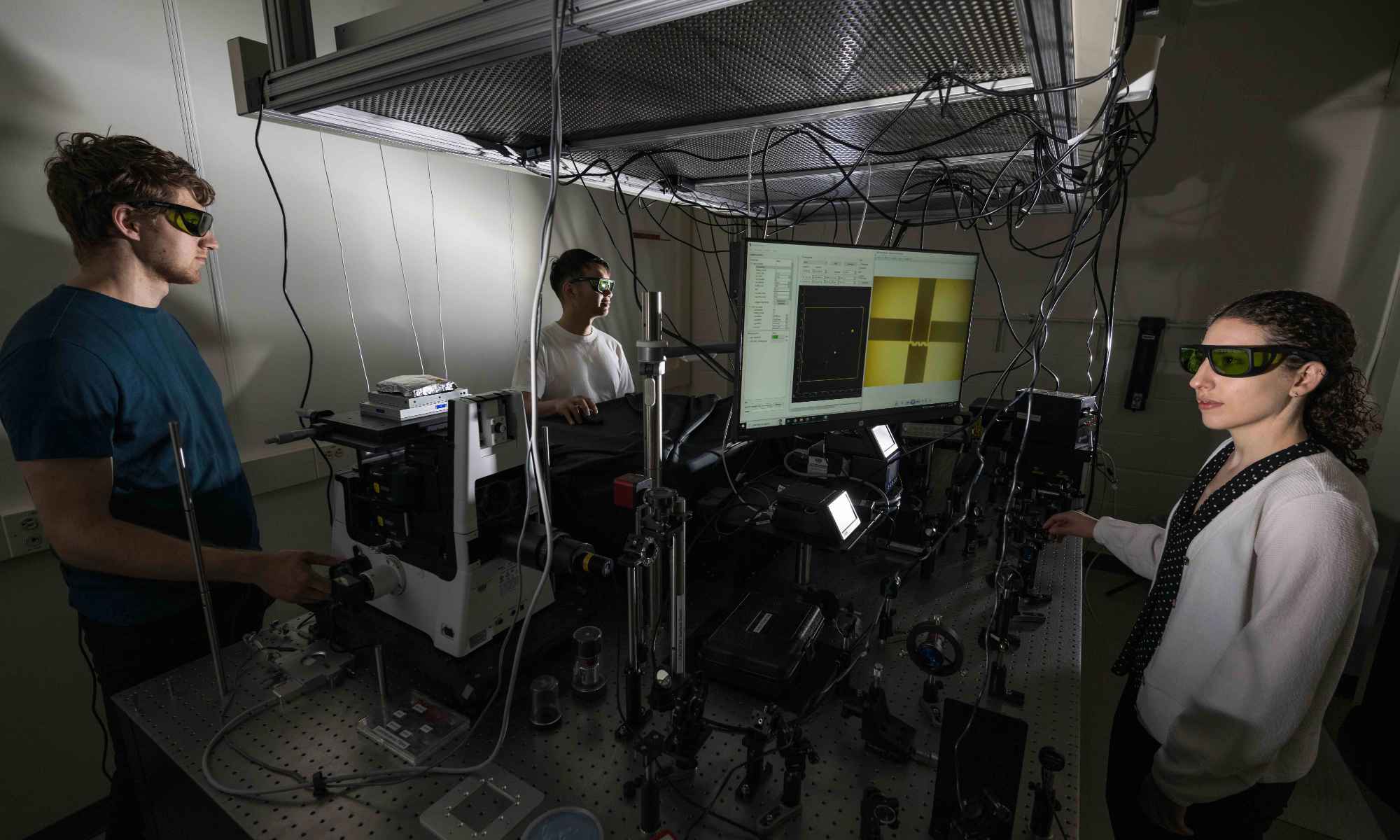
Extracellular vesicles could provide early detection of diseases such as cancer. But to analyze EVs, scientists first need to catch them.
Researchers from the University of Rochester and University of Chicago teamed up for one of the first known projects to successfully isolate and study extracellular vesicles (EVs).
Extracellular vesicles are tiny particles—as small as 40 nanometers in diameter—released by cells into the bloodstream and other fluid-filled cavities. EVs carry proteins, lipids, metabolites, and genetic material unique to the cells that release them. As a result, they could serve as valuable biomarkers for the early detection of diseases, including cancer—especially if single EVs could be assessed individually.
To this end, the researchers adapted nanomembranes from the lab of James McGrath, a professor of biomedical engineering at the University of Rochester, in a microfluidic cross-flow filtration system to capture and study individual EVs. Their findings appear in Communications Biology.
‘First foray’ into functionality of extracellular vesicles
“If the vesicles carried important genetic material, you’d want a fairly stable pH in that environment,” says Deborah Nelson, a professor of pharmacological and physiological sciences at Chicago. “So, we wanted to ask a basic question: Are there proteins in the vesicle surface that could be responsible for maintaining a stable pH environment in the inside of the vesicle?”
Once the researchers were able to stabilize the vesicles on a membrane in the filtration system, they could look at them with a powerful microscope and measure changes in the vesicles’ pH levels over time or in response to changes in the solution. As a result, Nelson and Vladimir Riazanski, a research associate professor in her lab, discovered that the EVs have a transporter protein on their surface called a sodium hydrogen exchanger, common to all epithelial cells.
“Once you have a mechanical platform that allows you to study function in a quantitative way, now we can look at how you might be able to recognize altered EVs and isolate certain subsets of the population,” Nelson says. “This was a first foray into understanding their functions at the single vesicle level.”
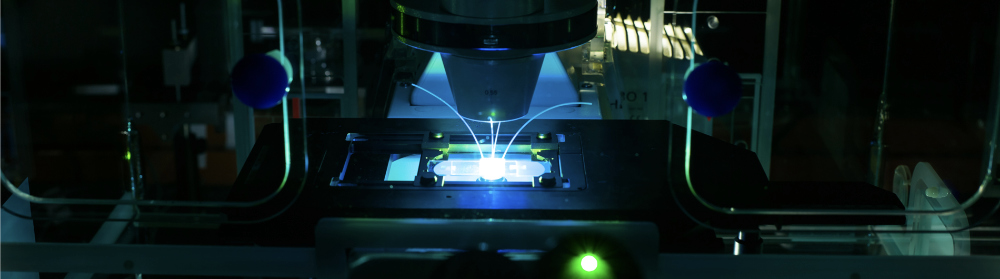
Catch EVs as you can
McGrath’s lab has pioneered the development of ultrathin membranes—just 100 nanometers thick—that are made from silicon nitride. The membranes contain billions of tiny pores, which can capture EVs and other tiny particles in microfluidics devices that circulate different fluids around the particles in a controlled manner and measure the responses.
“We’re particularly interested in the diagnostic potential of extracellular vesicles,” McGrath says. “Because tumor cells shed them abundantly, long before you ever manifest symptoms, these small clues are floating in your bloodstream as potential biomarkers. Our materials have the ability to catch and isolate individual EVs because the membrane pores and EVs are about the same size.”
McGrath is excited that the collaboration resulted in a groundbreaking application for the membranes. “Anytime our materials help a collaborator do first-of-a-kind science, it’s a big deal,” McGrath says. “This paper uses our materials to reveal an important detail in biology that has never been seen before.”
Produced by all types of cells in the body, EVs were originally thought to be like little garbage bags, carrying away junk from the cells. However, as researchers learned more, they began to see evidence that EVs affect cells around them by binding to receptors, initiating signal pathways, and even traveling over a distance to a target cell—which some researchers believe could play a role in cancer metastasis. And yet, a better understanding of extracellular vesicle functionality means that some of these same processes could eventually be harnessed to deliver targeted cancer-fighting therapies and drugs.
The National Institutes of Health and the Department of Defense supported the study. Additional authors include Gerardo Mauleon and Adriana M. Zimnicka from the University of Chicago and Kilean Lucas and Samuel Walker from the University of Rochester.
Read more
 ‘Organ on a chip’ is the wave of the future
‘Organ on a chip’ is the wave of the future
Rochester researchers are building technology to predict the course of tendon injuries—a form of personalized medicine that will lead to more effective treatments.
 Rochester to advance research in biological imaging through new grant
Rochester to advance research in biological imaging through new grant
A multidisciplinary collaboration will create a new light-sheet microscope on campus, allowing 3D imaging of complex cellular structures.
 Smaller is better for detecting biomarkers of trauma and cancer
Smaller is better for detecting biomarkers of trauma and cancer
Detecting tiny biomarkers circulating in our bodies is problematic and costly. Researchers are developing a cost-effective detection device using nanotechnology.