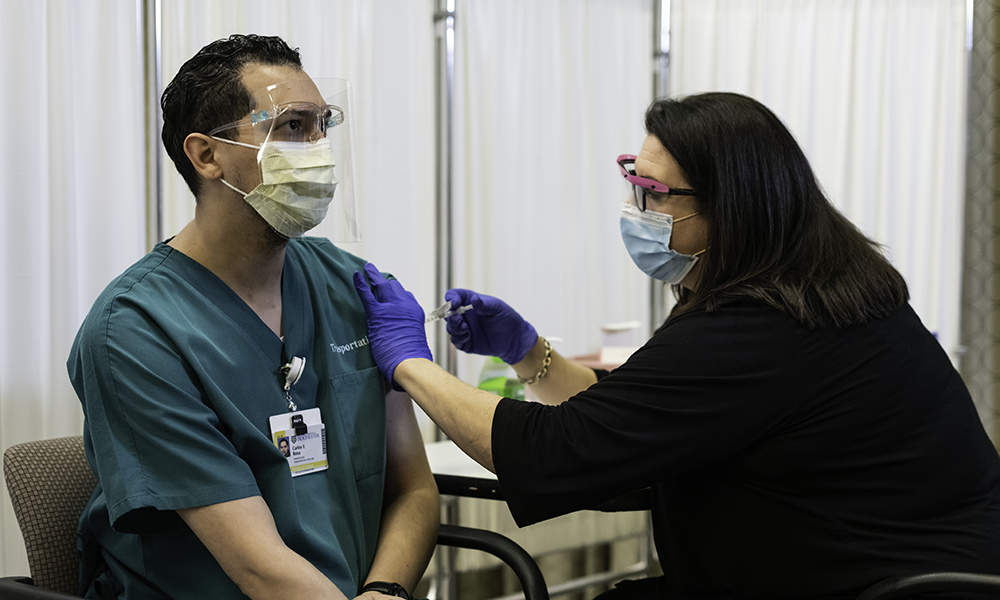
Researchers and volunteers in Rochester have been involved in the testing of the Pfizer/BioNTech vaccine since May.
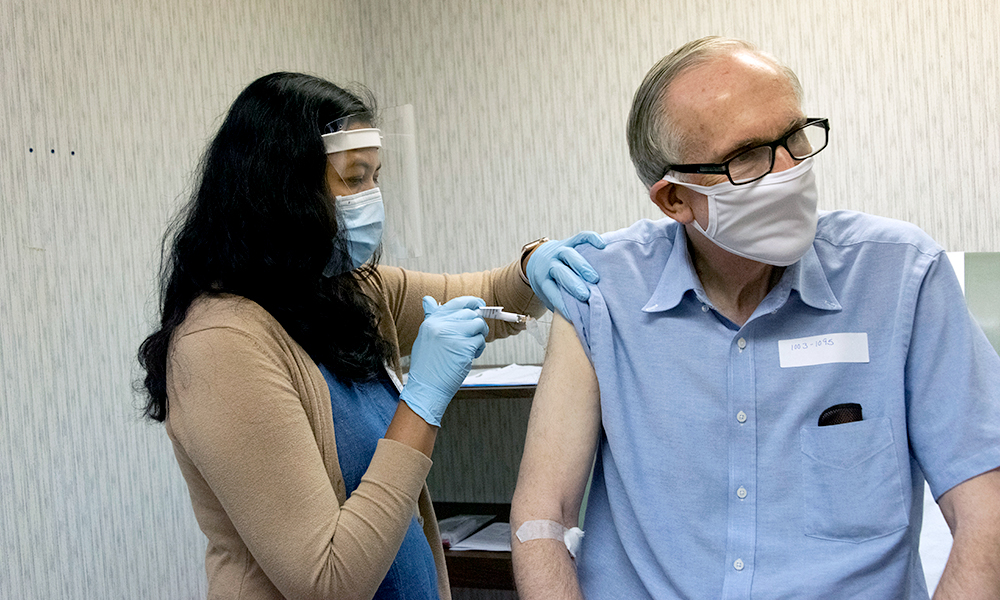
Late last week the Food and Drug Administration granted emergency use authorization to a COVID-19 vaccine developed by Pfizer and German pharmaceutical company BioNTech. A shipment of the Pfizer/BioNTech vaccine arrived at Rochester on Monday and was administered to 10 employees at Strong Memorial Hospital. The first person in Rochester to receive the COVID-19 vaccine, outside of a trial, was patient transport worker Carlos Rosa.
“Hope for the end to this pandemic is on the horizon.”
—Michael Apostolakos, chief medical officer, University of Rochester Medical Center
“We’ve had to share a fair amount of difficult news over the past 9 to 10 months and I’m delighted to join with my colleagues to share this great news today,” said Michael Apostolakos, chief medical officer at the University of Rochester Medical Center, in a press conference on December 15. “The Pfizer vaccine has proven safe and effective in rigorous scientific trials involving tens of thousands of persons. Hope for the end to this pandemic is on the horizon.”
Beginning December 16, the Medical Center will open a clinic that will administer the vaccine to 200 employees every four hours. Apostolakos said additional shipments of the vaccine will be rolled out in phases, and his best guess is that the general public would be able to receive the vaccine between June and August of 2021.
“I believe we’ll get enough supply that we’ll get through the health care workers, get through the patients with co-morbid conditions, get through nursing home and nursing home staff, and sometime June to August, we’ll get to the public,” he said. “But that depends on supply and some things we can’t control.”
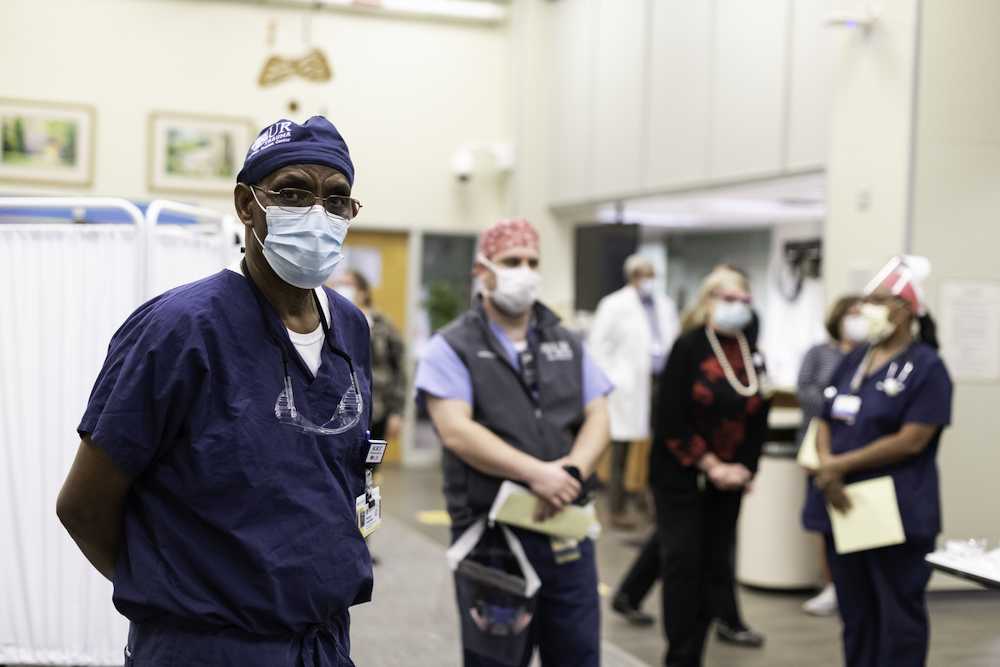
Researchers and volunteers in Rochester have played a pivotal role in the testing of the Pfizer/BioNTech vaccine since May, when the first human studies were launched as part of a phase 1 clinical trial. In a collaboration between the Medical Center and Rochester Regional Health, Rochester’s trial was led by Edward Walsh, a professor of infectious disease medicine at the Medical Center and a member of the Infectious Disease Unit at Rochester General Hospital (RGH) and Ann Falsey, a professor of infectious disease medicine, co-director of the Medical Center’s Vaccine and Treatment Evaluation Unit, and a member of the Infectious Disease Unit at RGH.
Rochester was one of only four sites in the US involved in this phase 1 study of the Pfizer/BioNTech vaccine. That trial evaluated four variations of the vaccine and the version that was tested in Rochester was selected to move into phase 3 trials in July. Volunteers in Rochester were the first in the nation to be dosed in the phase 3 clinical trial of the vaccine. Phase 3 marks the final stage of vaccine development prior to FDA approval, mass production, and distribution.
“No other vaccine in history has been given this much attention or this much work,” Apostolakos said. “It wasn’t that corners were cut with developing the vaccine, it was that so much more effort was made to do all the steps but in a very concise way that enabled us to get this out. It’s a modern miracle of 2020; this couldn’t have happened in 1920, but we’re able to get it out. It’s safe and effective and we’re going to encourage people to take it.”
The Pfizer/BioNTech coronavirus vaccine study can trace its roots back to decades of research conducted at Rochester to improve the efficacy of vaccines and develop new vaccines against Hib, a bacterium that causes meningitis and pneumonia, and HPV, the virus that causes cervical cancer.
The research behind the Hib vaccine led to the creation of Praxis Biologics, which was formed by Medical Center pediatrician David Smith in the 1980s as a start-up company. Praxis Biologics was eventually acquired by Pfizer and many of the scientists who worked at Praxis moved to Pfizer and helped establish the company’s vaccine development division. The team at Pfizer continued to collaborate with researchers in Rochester. Subsequent technological and scientific advances made by Rochester researchers studying RSV, a respiratory virus, and seasonal coronaviruses, contributed to Pfizer/BioNTech’s current COVID-19 vaccine.
“It’s wonderful to know that some of the scientists who designed and have overseen the Pfizer research that has demonstrated that this vaccine works, live and work here in Rochester,” said Robert Mayo, chief medical officer at Rochester Regional Health. “We can take pride in the fact that our leaders in this community have contributed to a worldwide vaccine that will change the course of this pandemic.”
Read more
 FDA Committee Votes to Approve Emergency Use of Pfizer Coronavirus Vaccine
FDA Committee Votes to Approve Emergency Use of Pfizer Coronavirus VaccineThe development of the vaccine can trace its origins to decades of infectious disease research conducted at Rochester.
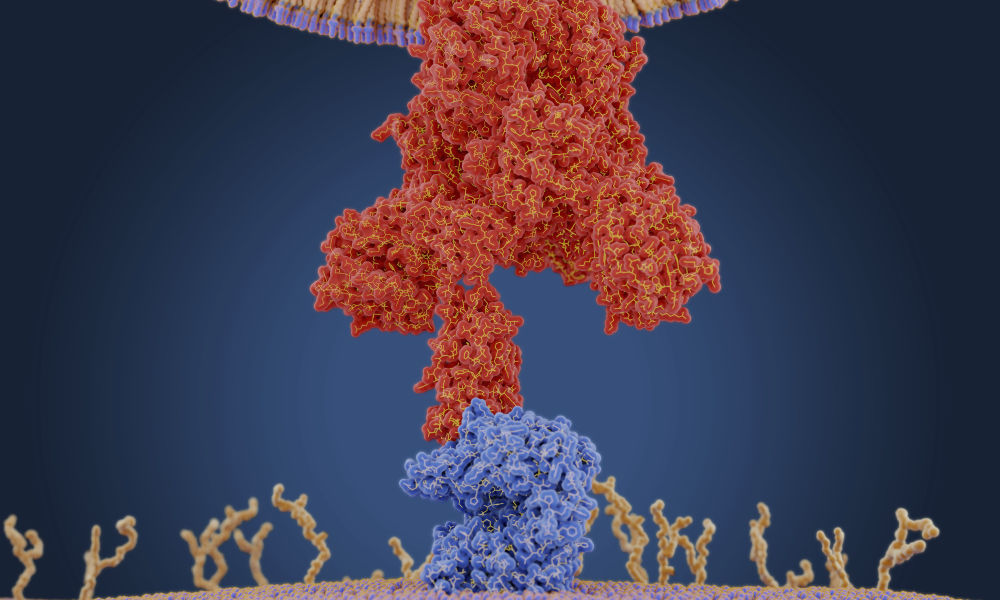 COVID-19 vaccine: What’s RNA research got to do with it?
COVID-19 vaccine: What’s RNA research got to do with it?RNA research at the University of Rochester provides an important foundation for developing antiviral drugs, vaccines, and other therapeutics to disrupt the global spread of coronavirus.
 Phase 3 AstraZeneca Coronavirus Vaccine Study Reopens at URMC
Phase 3 AstraZeneca Coronavirus Vaccine Study Reopens at URMCThe University of Rochester Medical Center is taking part in a phase 3 clinical trial for a potential coronavirus vaccine being developed by AstraZeneca and the University of Oxford.