Rochester biologists who study the genetics of lifespan suggest novel targets to combat aging and age-related diseases.
Natural selection produces mammals that age at dramatically different rates. Take, for example, naked mole rats and mice. The former can live up to 41 years, nearly ten times as long as similar-size rodents such as mice.
What accounts for longer lifespan? According to new research from University of Rochester biologists, mechanisms controlling gene regulation are a key piece of the puzzle.
In a paper published in Cell Metabolism, the researchers, including Vera Gorbunova, the Doris Johns Cherry professor of biology and medicine; Andrei Seluanov, professor of biology and medicine; and Jinlong Lu, a postdoctoral research associate in Gorbunova’s lab and the first author of the paper, investigated genes connected to lifespan. Their research uncovered specific characteristics of these genes and revealed that two regulatory systems controlling gene regulation are critical to longevity. The findings help scientists better understand longevity and may provide new targets to combat aging and age-related diseases.
Comparing gene regulation patterns
The researchers compared the gene regulation patterns of 26 mammalian species with diverse maximum lifespans. The lifespans ranged from two years (shrews) to 41 years (naked mole rats). The researchers then identified thousands of genes related to a species’ maximum lifespan. These genes were either positively or negatively correlated with longevity.
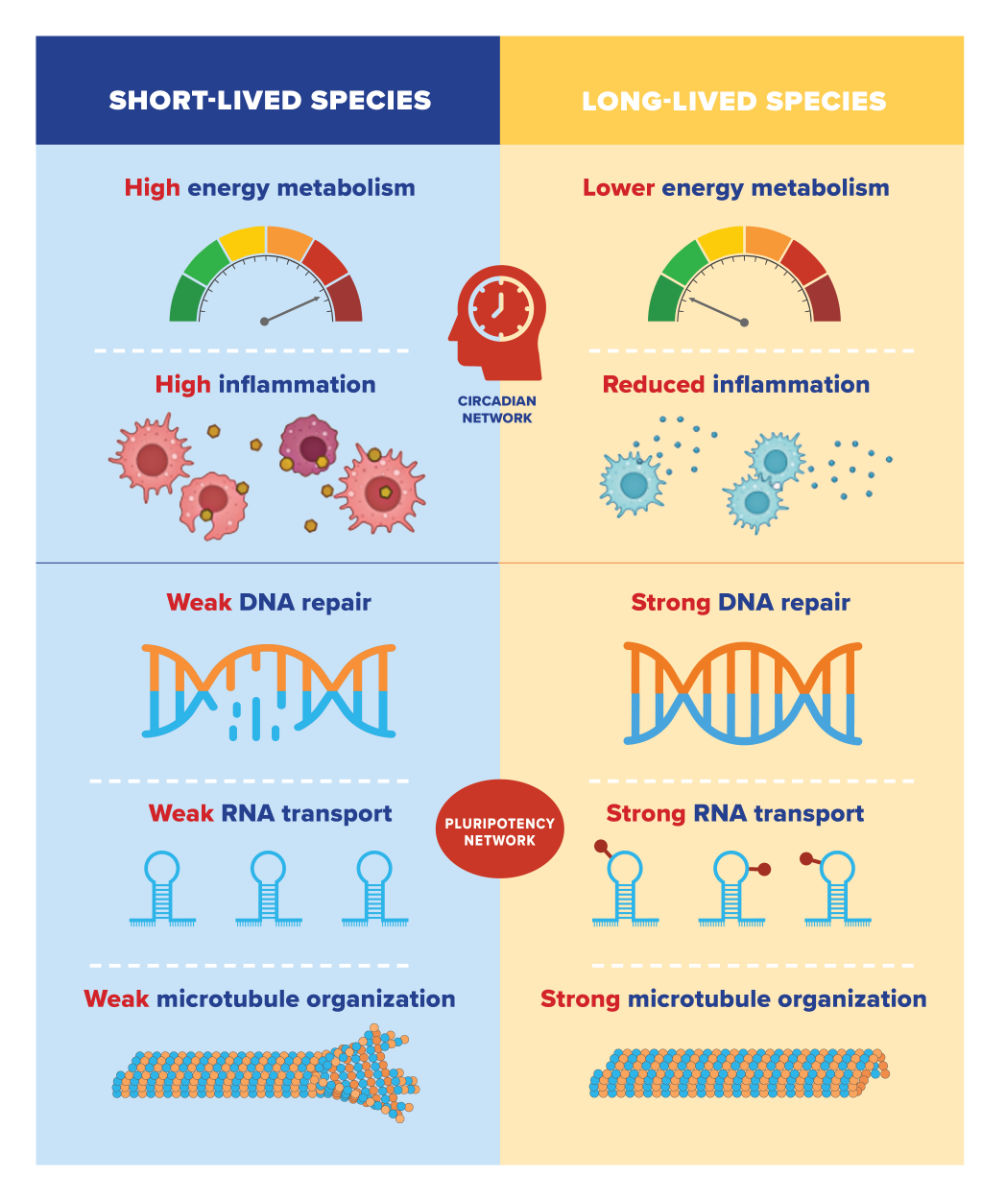
They found that long-lived species tend to have low expression of genes involved in energy metabolism and inflammation. However, they have high expression of genes involved in DNA repair, RNA transport, and organization of cellular skeleton (or microtubules). Gorbunova and Seluanov showed in previous research that mammals with long lifespans have features such as more efficient DNA repair and a weaker inflammatory response.
The opposite was true for short-lived species. Short-lived species tend to have high expression of genes involved in energy metabolism and inflammation. They have low expression of genes involved in DNA repair, RNA transport, and microtubule organization.
Two pillars of longevity
When the researchers analyzed the mechanisms that regulate these genes, they found two major systems at play. Circadian networks control the negative lifespan genes—those involved in energy metabolism and inflammation. That is, the genes’ expression is limited to a particular time of day. This may help limit the overall expression of the genes in long-lived species.
This means we can exercise at least some control over the negative lifespan genes.
“To live longer, we have to maintain healthy sleep schedules and avoid exposure to light at night as it may increase the expression of the negative lifespan genes,” Gorbunova says.
On the other hand, the pluripotency network controls positive lifespan genes—those involved in DNA repair, RNA transport, and microtubules. The pluripotency network helps in reprogramming somatic cells—any cells that are not reproductive cells—into embryonic cells. Embryonic cells can more readily rejuvenate and regenerate, by repackaging DNA that becomes disorganized as we age.
“We discovered that evolution activated the pluripotency network to achieve longer lifespan,” Gorbunova says.
The pluripotency network and its relationship to positive lifespan genes is therefore “an important finding for understanding how longevity evolves,” Seluanov says. “Furthermore, it can pave the way for new antiaging interventions that activate the key positive lifespan genes. We would expect that successful antiaging interventions would include increasing the expression of the positive lifespan genes and decreasing the expression of negative lifespan genes.”
Read more
 ‘Longevity gene’ responsible for more efficient DNA repair
‘Longevity gene’ responsible for more efficient DNA repairRochester researchers uncover more evidence that the key to the “Fountain of Youth” may reside in a gene that is found to produce more potent proteins in species with longer lifespans.
 Connecting the dots between aging, Alzheimer’s, and ‘junk DNA’
Connecting the dots between aging, Alzheimer’s, and ‘junk DNA’Biologists Vera Gorbunova and Andrei Seluanov join colleagues at Brown and NYU in the quest to find potential targets of treatments and therapeutics for neurodegenerative diseases.
 Why do naked mole rats live long, cancer-free lives?
Why do naked mole rats live long, cancer-free lives?Rochester biologists were surprised to see that despite its remarkable longevity, the naked mole rat still has cells that undergo senescence, like the cells in much shorter-lived mice.