Document Management in Click IRB
Institutional Review Boards (IRBs) conduct their reviews and make determinations based primarily on the documents provided by the study team within a submission (e.g., study protocol, consent documents, recruitment materials, study assessments). Responses to questions in an IRB submission should similarly be based on the content of the corresponding study documentation.
Appropriate management of study documents within an IRB review system is therefore critical for facilitating the review process. Keep the following ‘golden rules’ in mind when utilizing Click IRB.
Rule #1: Maintain the document audit trail
An audit trail provides a chronological record of all changes or transactions within a given system or database. Like the audit trail of a bank record that documents the amount and timing of every deposit or withdrawal over time, online IRB review platforms (including Click IRB) record information every time a document is uploaded into a submission form and every time a response to a submission form question is provided (or changed). Maintaining this information is necessary because it documents the history of the submission and it allows the IRB to quickly and easily identify changes from one version of a submission or document to the next.
While there is programming built into Click IRB that maintains the audit trail ‘behind the scenes’, the accuracy of that audit trail is only as accurate as the user’s actions. To maintain an accurate audit trail of a document in Click IRB, users must correctly ‘layer’ revised versions of existing documents. This is done utilizing the ‘Update’ button adjacent to the existing document.
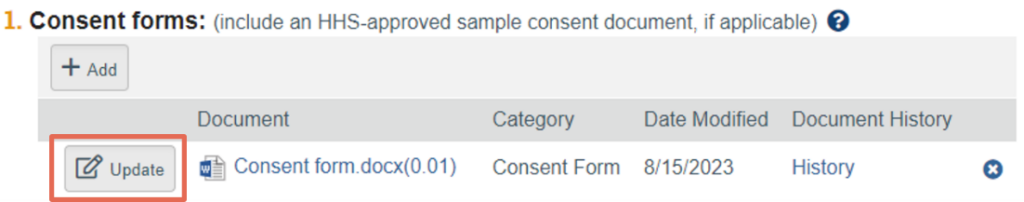
Adding revised documents via the ‘Add’ option and deleting old versions does not maintain the audit trail; the ‘Add’ button should only be used when adding new documents into the system, not previously reviewed documents.
In other words, think of a Click IRB submission as a traditional paper filing cabinet. Each smart form in the submission is one of the drawers and each field on the form, including each document within a field, is a separate file folder in the drawer. In this scenario, the audit trail of a given field or document is maintained in their respective file folder. Every time a field or document is revised, the revised version is added to the field’s/document’s respective file folder in front of (or on top of) the old version – this is the equivalent of using the ‘Update’ button to upload a revised version of a specific document. Whereas, filing a revised document in a brand new folder (hence breaking the audit trail) is the equivalent of using the ‘Add’ button.
Rule #2: Use consistent, short, but descriptive file naming conventions
When a document is uploaded into Click IRB, users are given the option to identify a file name.
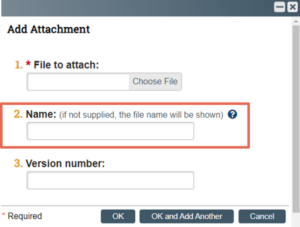
The name identified in the field then pulls through to the submission form and, later (upon IRB approval) to the submission approval letter. The naming convention used to identify the document should allow reviewers (as well as any individuals that might view IRB approval letters – e.g., Study Monitors, Food & Drug Administration Inspectors) to quickly and easily identify the type and purpose of the document. This is particularly important when a submission includes a large number of documents (e.g., multiple consent documents and recruitment materials).
Naming conventions should be short (less than 25 characters) but descriptive, and consistent across document versions. For example:
| Version 1 | Version 2 |
|---|---|
| Protocol.v1 | Protocol.v2 |
| STUDY1234_Protocol_v1 | STUDY1234_Protocol_v2 |
| Consent_CohortA_v1 | Consent_CohortA_v2 |
| 1234_Consent_05MAR2020 | 1234_Consent_11FEB2021 |
When no name is assigned to the document, which is often the case, Click IRB will default to the file name of the document. Consequently, study teams should be mindful of the naming convention recommendations above (i.e., consistent and short but descriptive) when they create and/or modify study document files in their word processing software. In other words, when a document is created by a study team, if the name applied to the document file is appropriately named when the document is saved in MS Word, for example, the appropriate file name will pull into Click IRB automatically when/if the document name field is left blank in Click IRB.
Rule #3: Use consistent versioning methods
Similar to document names, Click IRB also gives users the option to identify an alphanumeric version number when a document is uploaded into the system.
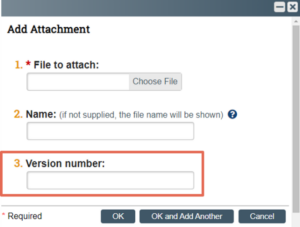
A version date, version number or a combination of a version date and number may be entered. The version identified in the field also pulls through to the submission form and approval letter, as with the document name. When the field is left blank, Click IRB will automatically assign a version number each time a revised version of the document is uploaded.
Ideally, the version identified in Click IRB should be consistent with whatever version, if any, is identified in the document itself (e.g., in the document footer). This is particularly true for sponsored research; sponsors and monitors will often require that the approval letter identify the version of an approved document. However, the accuracy of the version that appears in the approval letter is only as accurate as what is entered into the system by the study team.
Ultimately, whichever version method is utilized – allowing Click IRB to create a default version or identifying a version date consistent with what is identified in the document itself – it should be consistently applied across all revisions or modifications throughout the life of the research. Inconsistent versioning will result in an unclear document history (e.g., if a protocol is versioned with a date in one insistence [28JAN2021] and a number in a second instance [v.1.4], based on the version formatting, there is no way to determine the chronology of the document).
Key tips
As referenced in the second ‘golden rule’, these practices ultimately apply to how a study team should organize their own study files. Although file organization, naming conventions, and versioning might seem like they’re only for Type A personalities or the organization-obsessed, failing to maintain study files in a manner that is not clear, orderly and consistent can have tangible consequences on the conduct of the research. Consider, for example, what happens when a study team member can’t readily identify the current approved version of a protocol and they need to reference a schedule of assessments, verify eligibility requirements, determine whether a research event needs to be reported according to a data and safety monitoring plan, or revise the protocol. Each scenario could potentially result in exposing a subject to unnecessary risk and non-compliance.
Principal Investigators (PIs) are responsible for maintaining documentation that demonstrates their compliance with applicable federal regulations and institutional policy, as well as the study protocol (reference OHSP Policy 901: Investigator Responsibilities). PIs cannot rely on Click IRB (or any other IRB review platform) to maintain documentation of their IRB approval letters and corresponding IRB-approved study documents. All necessary study documentation should be saved by the PI/study team as soon as a submission is approved or acknowledged. Reference what types of documentation must be maintained by the PI; guidance is also available through the OHSP’s Study Documentation Templates and Post-Approval Consultation.
