Accessing and Utilizing Watermarked Documents
Institutional Review Boards (IRBs), including the Research Subjects Review Board (RSRB), routinely apply a watermark to consent forms and recruitment materials at the time of IRB approval (including initial approvals, as well as modifications and continuing reviews).
IRBs typically further require study staff to utilize the current, watermarked version of these documents during the recruitment and informed consent process. In other words, the current, watermarked versions are the only versions of the documents the IRB recognizes as valid (the watermark validates IRB-approval of the document’s contents).
What is required?
Requirements related to what documents are watermarked and when study teams are required to use them, will depend on the Reviewing IRB. Generally however, it’s safe to assume that if a document is watermarked by the IRB then the study team is required to utilize the current, watermarked version of that document during study conduct.
Locally, the RSRB watermarks all consent forms and recruitment materials. OHSP Policy 901: Investigator Responsibilities requires all investigators to obtain informed consent utilizing the current, watermarked version of the document.
Similarly, watermarked versions of recruitment materials should be utilized when appropriate, based on the nature of the materials (reference OHSP Guideline for Recruitment Methods and Materials). E.g., printed flyers posted in public locations should include the watermark, whereas language approved for a social media post would not need to include the watermark as doing so would not be feasible.
OHSP Policy 901 Investigator Responsibilities further requires study teams to ‘maintain documentation to support RSRB determinations…’ This means that study teams should maintain documentation of all IRB-approved materials, including all versions of any watermarked documents.
Reference our Study Documentation Requirements page for more information.
What does the watermark look like?
The format of an IRB watermark will vary by IRB. The RSRB’s watermark appears in the lower right-hand corner of an approved document and minimally includes the RSRB approval date. Based on the type of document, the watermark may further include an expiration date. For additional information, about expiration dates, reference our Click IRB FAQs.
How is the watermark applied?
Watermarks are routinely built into the Reviewing IRB’s approval process. RSRB watermarking occurs via programming within the Click IRB platform, based on the document category selected by the study team when a document is uploaded. For example, when a document is uploaded and the document is identified as a consent form in the category field, the document will be watermarked with both an approval and expiration date when the submission is processed for approval. Whereas, if the document is identified as an information sheet, it will only be watermarked with an approval date at the time of approval.
As the process is automated, RSRB staff do not have the ability to move or adjust where the watermark appears. The watermark will always appear in the lower right-hand corner of the document. As such, study teams should format consent and recruitment materials with at least a 1-inch footer and leave the lower right-hand footer of all consent and recruitment documents blank (RSRB consent form templates are already formatted to accommodate the watermark). Any content (e.g., page numbers or version dates) included in this area will be covered by the watermark, once it is applied.
In the event, a consent/recruitment document watermark is missing or does not appear to be accurate, contact the IRB Coordinator.
Where can watermarked documents be accessed?
For research reviewed by the RSRB, all approved study team members and individuals listed on the study’s guest list can access watermarked documents as follows:
Current watermarked documents
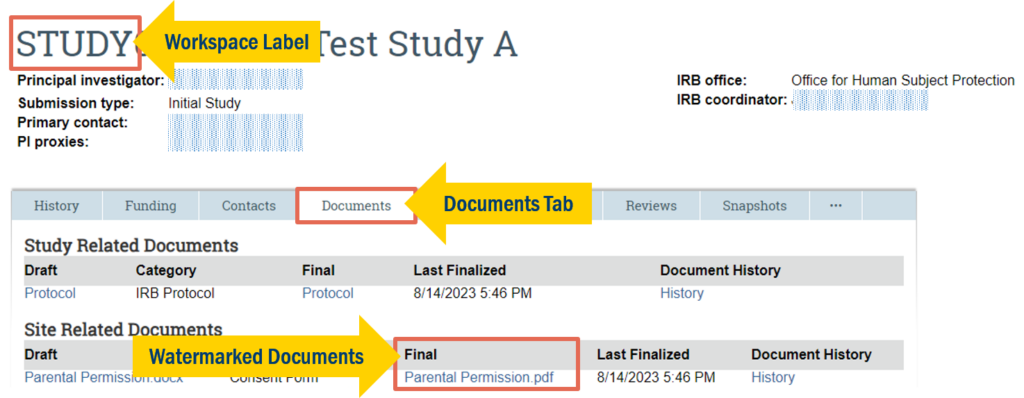
All current watermarked RSRB-approved documents are available on the ‘Documents’ tab in the STUDY workspace (labeled ‘STUDY0000…’; reference the image below). Watermarked versions are available under the ‘Final’ heading.
Historic watermarked documents
Historic (outdated) watermarked RSRB-approved documents are available on the ‘Documents’ tab of the applicable follow-on submission workspace (labeled based on the submission type, e.g., ‘MOD0000…’, ‘CR0000…’, ‘MODCR0000…’). For example, the documents approved with Modification #1 are available on the ‘Documents’ tab in the workspace of Modification #1.
Exception: Once a follow-on submission is approved (e.g., a modification or continuing review), the watermarked documents approved with the original RSRB approval are no longer accessible. This occurs because the documents approved with a follow-on submission copy to the main STUDY workspace to replace the previously approved documents. Study staff must be cognizant of downloading and maintaining the documents approved with the original RSRB approval in a timely manner (i.e., prior to submission of the first follow-on submission).
For research reviewed by an External IRB, access will depend on the Reviewing IRB.
